N-benzenesulfonyl-3-acetyl indole acyl hydrazone compound, preparation method and application
A technology of acetyl indolehydrazone and benzenesulfonyl, applied in the field of organic synthesis, to achieve the effect of reducing product loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation method of formula a-1 compound is:
[0048] 3mmol of AlCl 3 Add to a 50mL flask, add 5mL of dichloromethane, then add 1.5mmol of acetyl chloride at room temperature, react for 15min, add dropwise N-benzenesulfonyl indole dissolved in dichloromethane, react at room temperature for 2h, and detect by TLC After the reaction was complete, 10 mL of water was added to terminate the reaction, and extracted with dichloromethane (20 mL×3 times), the organic phases were combined, washed successively with saturated sodium bicarbonate solution (10 mL) and saturated sodium chloride solution (10 mL), and anhydrous sulfuric acid dried over sodium, concentrated under reduced pressure to obtain the crude product, and used GF 254 Thin layer chromatography Silica gel prepares a 20cm×20cm thin layer chromatography plate, dissolves the crude product in a small amount of dichloromethane and loads the sample on the thin layer chromatography plate, develops and separates to obt...
Embodiment 2
[0059] The preparation method of compound 2 is: take compound of formula a-1 (0.5mmol, prepared as above), m-chlorobenzohydrazide (0.5mmol) in 50mL flask, and add 5mL absolute ethanol to make it dissolve, add dropwise ( 1-2 drops) of glacial acetic acid and reflux reaction, after the reaction lasted for several minutes (5-10min), a large amount of solids began to be produced, and TLC tracked and detected that the reaction of the raw materials was complete; placed at room temperature to make it completely crystallized, and the crude product was obtained by suction filtration under reduced pressure. The product was washed several times with refrigerated (-20°C) absolute ethanol to obtain pure compound 2.
[0060] The reaction formula for preparing compound 2 by formula a-1 compound is as follows:
[0061]
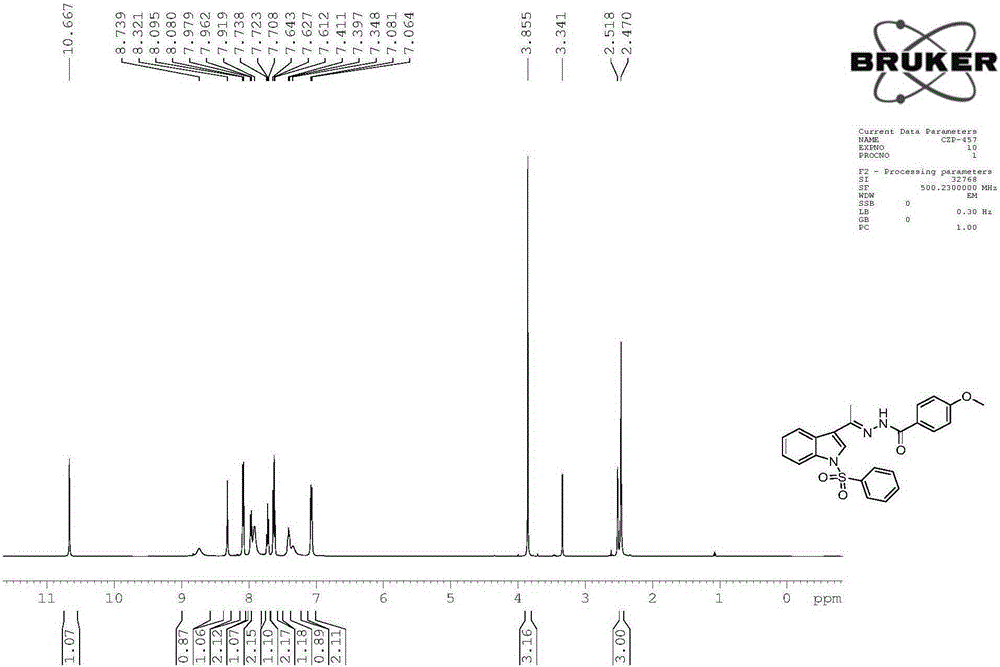
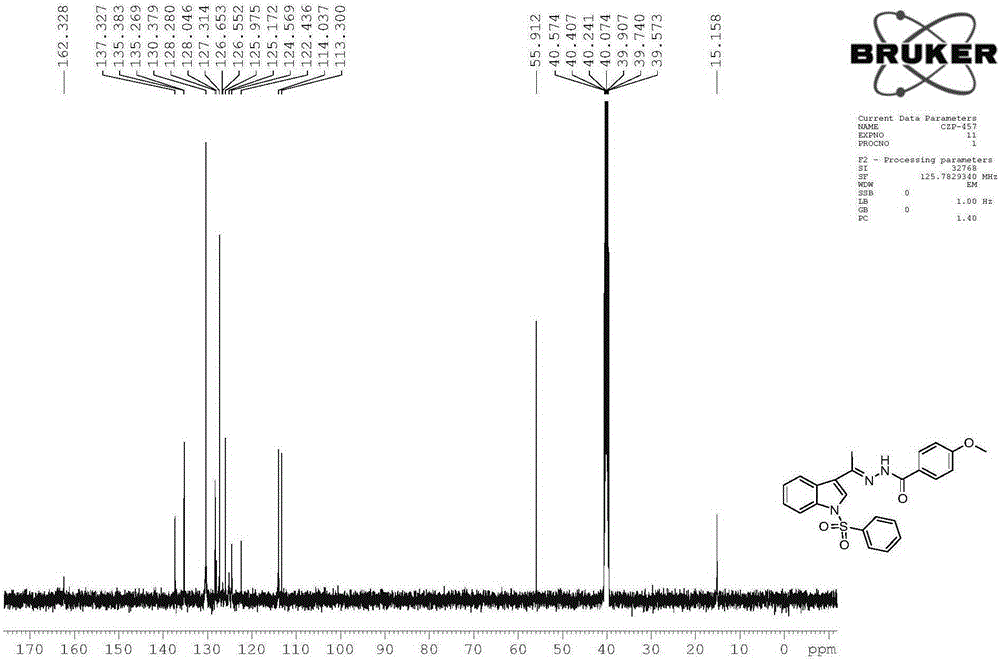
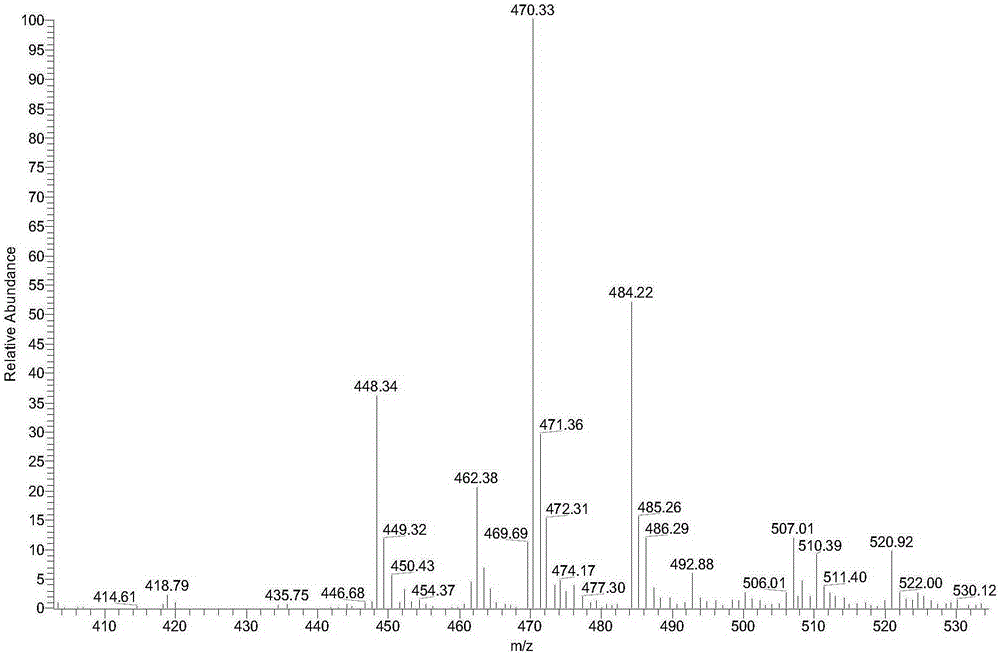
[0062] The physicochemical properties of compound 2 are as follows:
[0063] 1), white solid, melting point 196-198°C, yield 87%.
[0064] 2), the nuclear magnetic reson...
Embodiment 3
[0068] The preparation method of formula a-2 compound is:
[0069] 3mmol of AlCl 3 Add to a 50mL flask, add 5mL of dichloromethane, then add 1.5mmol of acetyl chloride at room temperature, react for 15min, add dropwise N-nitrobenzenesulfonylindole dissolved in dichloromethane, and react for 2h at room temperature 10mL of water was added to stop the reaction after TLC detection was complete, and extracted with dichloromethane (20mL×3 times), the organic phases were combined, and washed successively with saturated sodium bicarbonate solution (10mL) and saturated sodium chloride solution (10mL), Dry over anhydrous sodium sulfate, concentrate under reduced pressure to obtain crude product, use GF 254 Thin-layer chromatography Silica gel prepared a thin-layer chromatography plate of 20 cm × 20 cm, and the crude product was dissolved in a small amount of dichloromethane and then loaded on the thin-layer chromatography plate, developed and separated to obtain the compound of formula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com