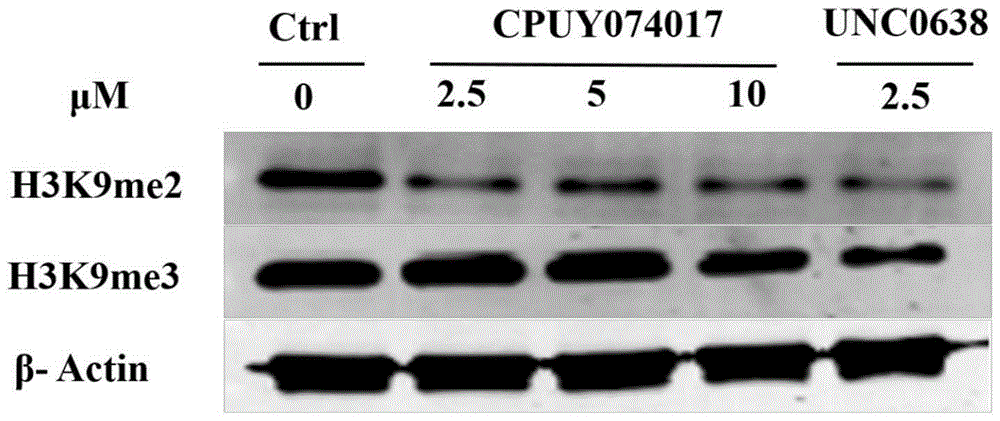
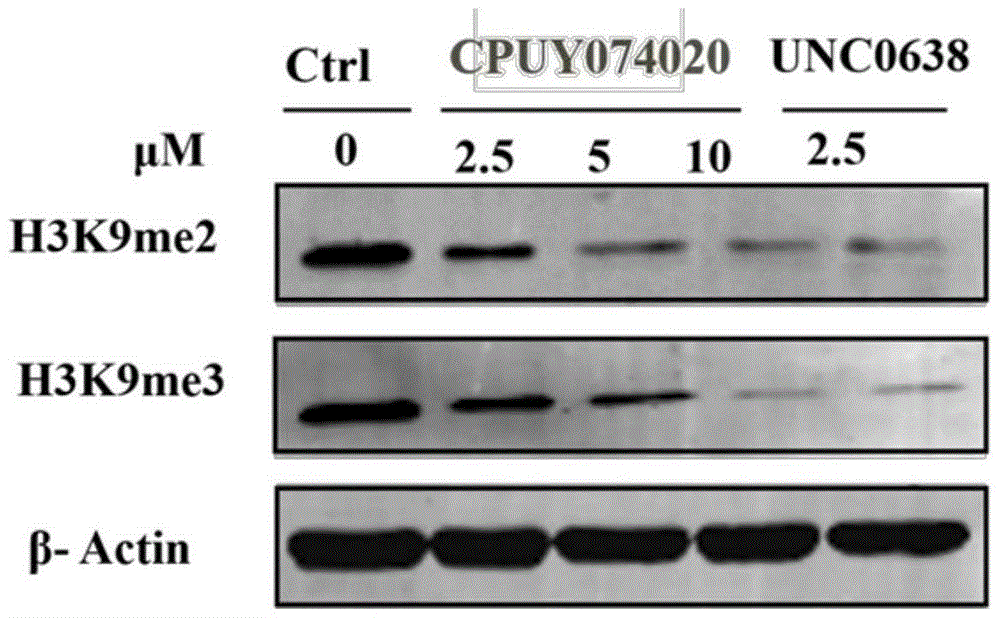
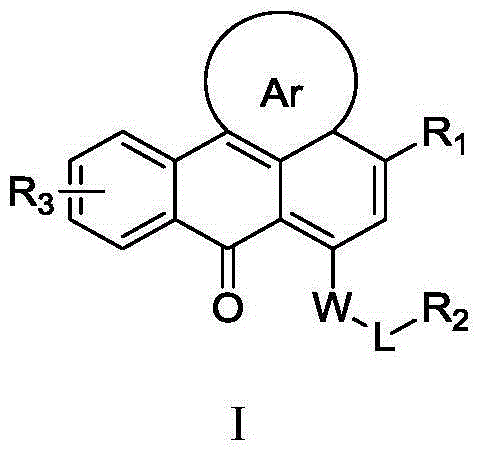
Heterocyclic anthracene ketone histone methyltransferase inhibitor and medical application thereof
A heterocycle and methoxy technology, applied in the field of medicinal chemistry, can solve problems that have not been studied clearly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation of 3,5-dibromo-6H-anthracene[1,9-cd]isoxazol-6-one
[0053]
[0054] 6H-Anthracene[1,9-cd]isoxazol-6-one (5g, 22.60mmol) was dissolved in AcOH (50mL), and bromine (2.90mL, 56.51mmol) in acetic acid solution was added dropwise to the reaction solution During TLC detection, the reaction was terminated. The reaction solution was adjusted to weak alkalinity with saturated sodium bicarbonate, extracted with dichloromethane (3×50 mL), the organic layer was dried with anhydrous sodium sulfate, the solvent was spin-dried, and the yellow 3,5-bis Bromo-6H-anthracene[1,9-cd]isoxazol-6-one (7.26g, 73%).m.p.247-248℃. 1 HNMR (300MHz, CDCl 3 ): δ8.45(d, J=7.71Hz, 1H, ArH), 8.08(d, J=7.5Hz, 1H, ArH), 7.97(s, 1H, ArH), 7.82(t, J=7.23Hz, 1H,ArH),7.72(t,J=7.35Hz,ArH).HRMS(ESI):calcdforC 14 h 5 Br 2 NO 2 [M+H] + 377.8760,found 377.8752.
Embodiment 2
[0056]
[0057] Differently substituted ammonia was dissolved in anhydrous aluminum chloride in dichloromethane solution, the compound of Example 1 was added to the mixture, stirred at 40° C. for 12 h, detected by TLC, and the reaction was stopped. The reaction was extracted with water (2 x 30 mL). The organic layer was dried with anhydrous sodium sulfate, and the solvent was spin-dried to obtain a red solid.
Embodiment 3
[0060] Preparation of 3-bromo-5-((4-methoxyphenyl)amino)-6H-anthracene[1,9-cd]isoxazol-6-one
[0061]
[0062] 3,5-dibromo-6H-anthracene[1,9-cd]isoxazol-6-one (0.10g, 0.26mmol) and p-methoxyaniline (0.19g, 1.58mmol) were synthesized according to the method in Example 2 . m.p.156-157℃. 1 HNMR (300MHz, CDCl 3 ):δ11.32(s,1H,NH),8.58(d,J=7.23Hz,1H,ArH),8.18(d,J=7.23Hz,ArH),7.82(t,J=7.47Hz,1H, ArH), 7.70(t, J=8.01Hz, 1H, ArH), 7.62(s, 1H, ArH), 7.30(d, J=8.79Hz, 2H, ArH), 7.05(d, J=8.85Hz, 2H ,ArH),3.91(s,3H,CH3).HRMS(ESI):calcdforC 21 h 13 BrN 2 o 3 [M+H] + 421.0182,found 421.0186.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com