Pseudovirions and preparation method and application thereof
A technology of pseudovirion and influenza A virus, which is applied in the field of pseudovirion and its preparation, can solve the problems of natural virus infection risk, RNA molecule instability, difficulty in meeting test requirements, etc., and achieve strong practical value and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
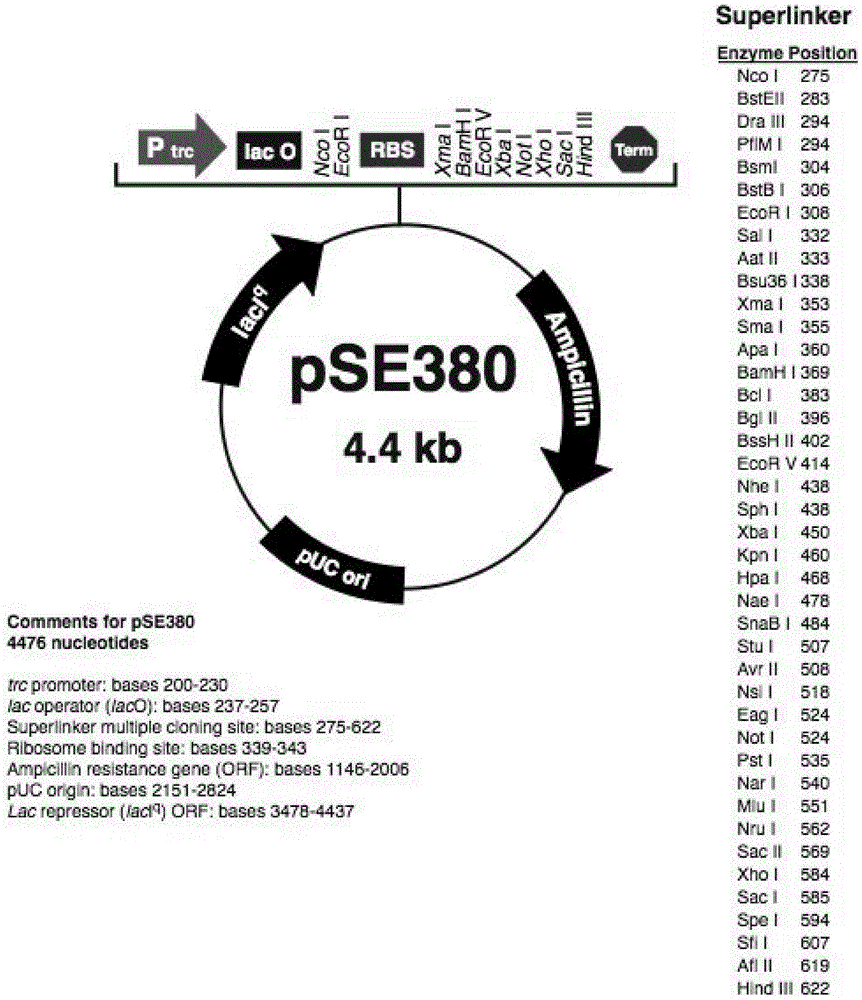
[0049] The present embodiment contains the preparation method of the pseudovirion of three kinds of detection fragments of influenza A virus, influenza B virus and β-actin in series, and its specific preparation steps are:
[0050] (1), construction of recombinant plasmid pSE380-MS2:
[0051] 1. According to the MS2 phage gene sequence (NC_001417) in the GenBank database, primers for PCR amplification of the envelope protein gene, maturation enzyme gene, and packaging signal sequence of MS2 bacteriophage were independently designed and artificially synthesized; the primers were: MS2L:CCTTTCGGGGTCCTGCTC (SEQ ID NO: 1) MS2BglIIR:GATTAGATCTGAGTTGAACTTCTTTGTTGTCTTC (SEQ ID NO: 2).
[0052] 2. Apply the above primers, use the MS2 phage gene as a template, and perform RT-PCR amplification according to conventional techniques to obtain a product of about 1780 bp, including the envelope protein gene, maturation enzyme gene, and packaging signal sequence of MS2 phage.
[0053] 3. Comb...
Embodiment 2
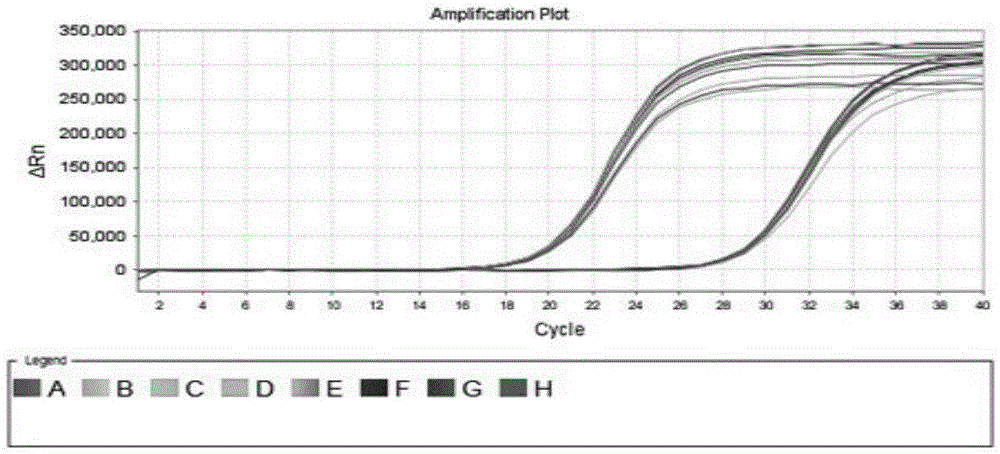
[0076] Example 2: Homogeneity Test of Virus-Like Particles as Strong Positive Control and Borderline Positive Control
[0077] Take 10 strong positive controls and 10 critical positive controls stored at -20°C, shake, mix and centrifuge, and take 50uL each for fluorescent PCR detection. The amplification system, amplification program, primers and probes are as follows.
[0078] RT-PCR amplification system:
[0079] 5×RT-PCRbuffer (final concentration)
1×
dNTP (final concentration)
0.6mM
[0080] Primer (used amount)
10pmol
Probe (Usage)
3pmol
Taq (consumption)
1U
MMLV (consumption)
50U
DEPC water
Make up to 20μl
(template)
(5μl)
[0081] The RT-PCR reaction procedure is as follows:
[0082]
[0083] Primers and probes are as follows:
[0084]
[0085] The Ct value of the fluorescent PCR result was used for statistical analysis to evaluate the uniformity of the str...
Embodiment 3
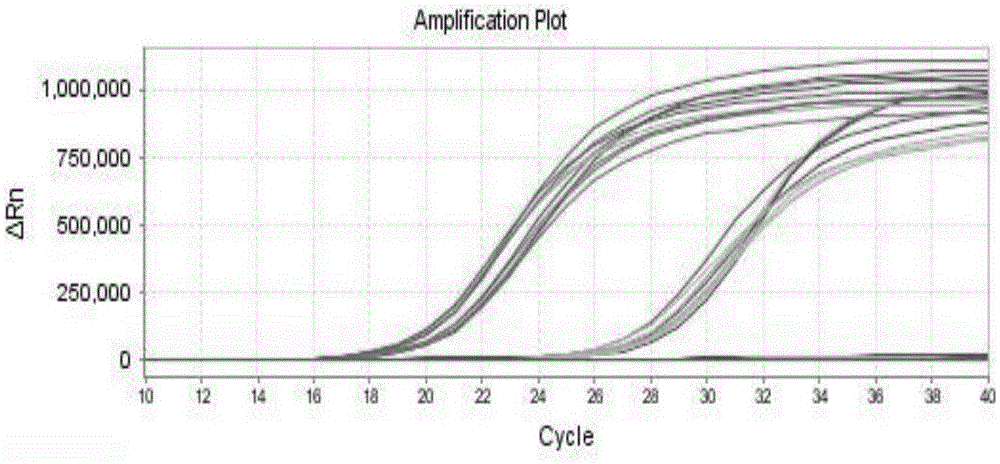
[0097] Example 3: Stability analysis of virus-like particles as strong positive control and critical positive control
[0098] Three tubes of the strong positive control and critical positive control stored at -20°C were taken at different time points for fluorescent PCR assay. For the detection method and the primers and probes used, see Example 2. The stability of each storage condition was statistically analyzed. Samples were taken once in the first month, and once a month from the sixth month onwards, and the fluorescent PCR assay and statistical analysis were performed. attached image 3It is the result graph of the stability test in October. The results of the 10 stability test data are shown in the following table:
[0099]
[0100] Strong positive control substance detects the regression analysis of variance of CT value (IA):
[0101]
[0102]
[0103] Strong positive control substance detects the regression analysis of variance of CT value (IB):
[0104]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com