Reducing responsive targeted polymeric micelle for mucus penetration and preparation method of polymeric micelle
A technology of polymer micelle and mucus penetration, applied in the field of reduction-responsive targeted polymer micelles and their preparation, can solve the problem of hindering the interaction between nanoparticles and focal cells, reducing the endocytosis effect of nanoparticles, and reducing intracellular drugs Slow and controlled release and other issues, to achieve the effect of good mucus permeability, low cytotoxicity and strong adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
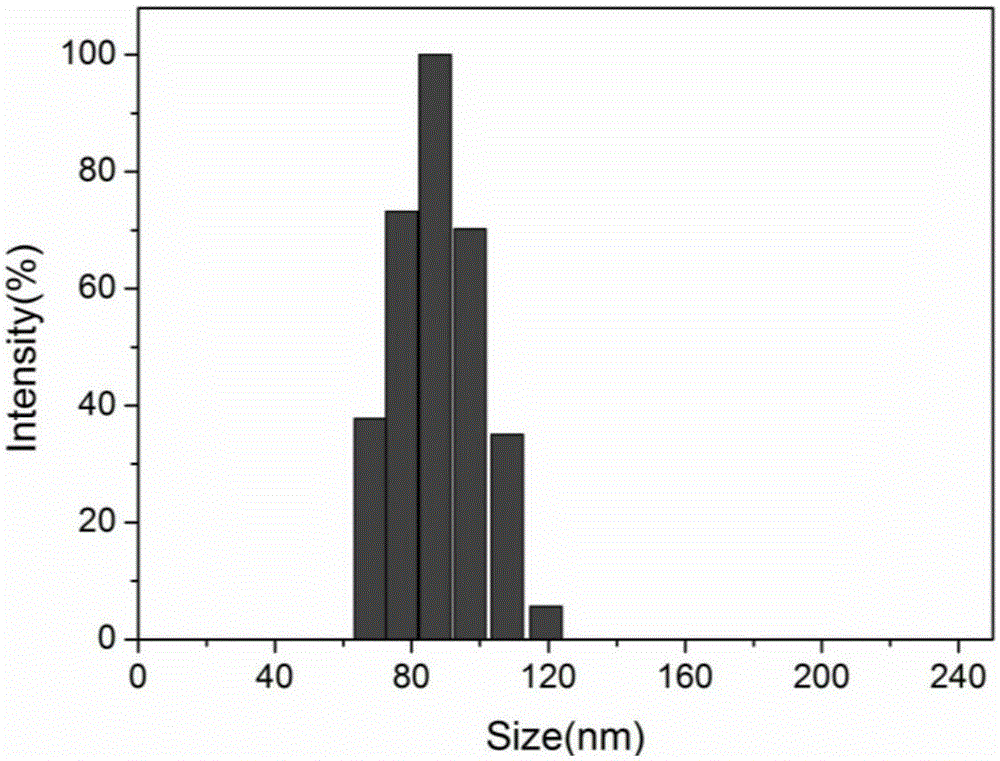
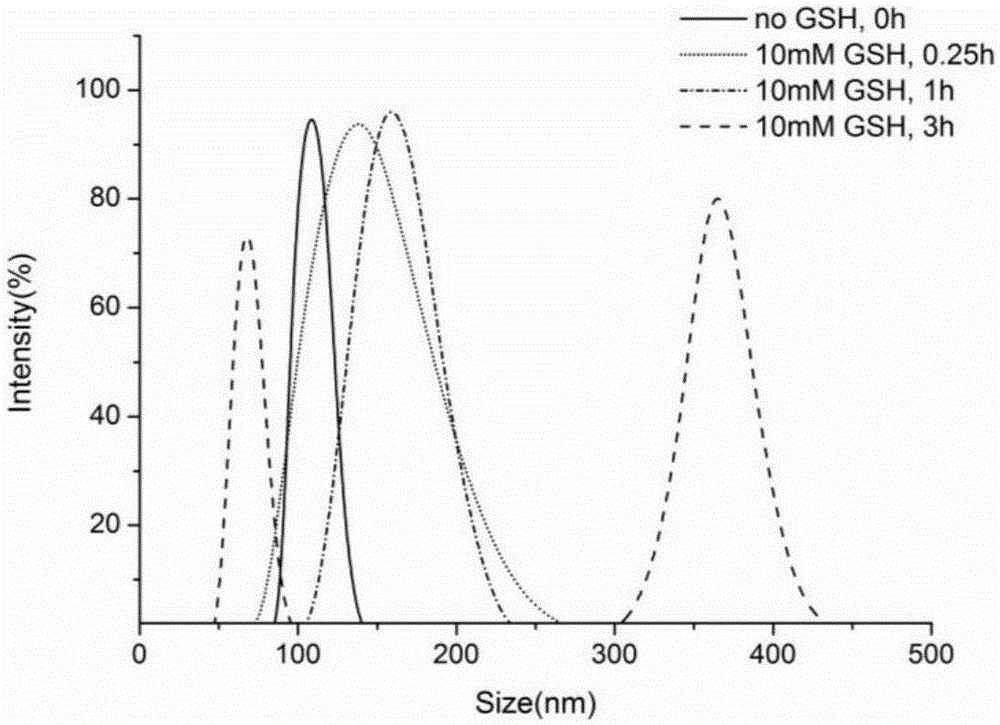
Image
Examples
preparation example Construction
[0033] A method for preparing a reduction-responsive amphiphilic polymer conjugate, the steps are as follows:
[0034] Poly(lactic-co-glycolic acid) (PLGA), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, 4-dimethylaminopyridine, 3,3'-disulfide Dipropionic acid is dissolved in N,N-dimethylformamide at a molar ratio of 1:(1.2~5):(0.6~2):(3~10), stirred and reacted at room temperature for 24~48h in the above solution Poly(lactic-co-glycolic acid) copolymer (PLGA):polyethylene glycol (PEG)=1:4 is dissolved in polyethylene glycol (PEG) by molar ratio, stirred at room temperature for 24-48 hours, post-treatment to obtain reduction-responsive amphiphilic Polymer combination.
[0035] The concentration of the PLGA dissolved in N,N-dimethylformamide is preferably 0.05-0.25 g / ml.
[0036] The specific steps of post-treatment are: dialyze the reaction solution in N,N-dimethylformamide for 12-24 hours, then dialyze in pure water for 24-48 hours, and freeze-dry to obtain the ...
Embodiment 1
[0044] Synthesis of reduction-responsive amphiphilic polymer conjugates.
[0045] Take polylactic acid-glycolic acid copolymer (PLGA, molecular weight is 10kDa) 1g, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 100mg, 4-dimethylaminopyridine 30mg, 85mg of 3,3'-dithiodipropionic acid was dissolved in 12ml of N,N-dimethylformamide, stirred at room temperature for 24 hours, and then 90mg of polyethylene glycol (PEG, molecular weight 1kDa) was added to the above solution , after stirring at room temperature for 24 hours, the reaction solution was dialyzed in N,N-dimethylformamide for 12 hours, then dialyzed in pure water for 24 hours, and then freeze-dried to obtain a reduction-responsive amphiphilic polymer conjugate.
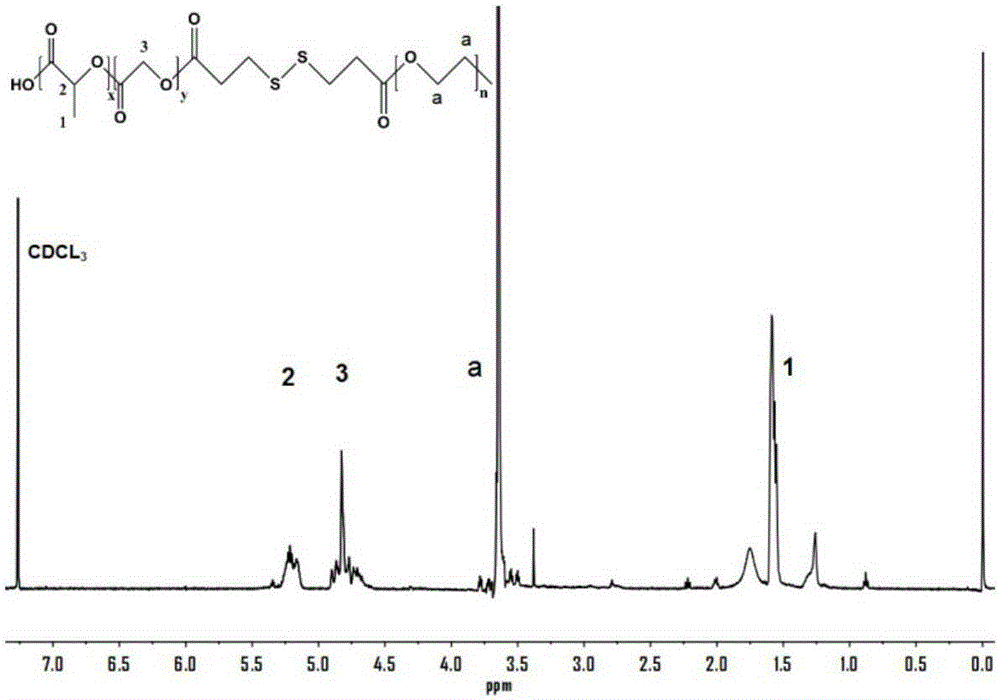
[0046] Such as figure 1 As shown in the H NMR spectrum, the characteristic peaks of PLGA and PEG can be obtained, which proves that the amphiphilic polymer conjugate is synthesized successfully.
Embodiment 2
[0048] Synthesis of reduction-responsive amphiphilic polymer conjugates.
[0049] Take polylactic acid-glycolic acid copolymer (PLGA, molecular weight is 21kDa) 0.4g, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 70mg, 4-dimethylaminopyridine 23mg , 65mg of 3,3'-dithiodipropionic acid, dissolved in 10ml of N,N-dimethylformamide, stirred at room temperature for 28 hours, then added polyethylene glycol (PEG, molecular weight 2.5 kDa) 25 mg, stirred at room temperature for 28 hours, dialyzed the reaction liquid in N,N-dimethylformamide for 14 hours, dialyzed in pure water for 26 hours, and freeze-dried to obtain a reduction-responsive amphiphilic polymer conjugate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Effective particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com