Ezetimibe tablets
A technology of ezetimibe and parts by weight, applied in the field of pharmaceuticals, can solve the problems of large release differences, human organ and tissue damage, side effects, etc., to reduce dissolution differences, reduce absorption fluctuations, and improve safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021]
[0022]
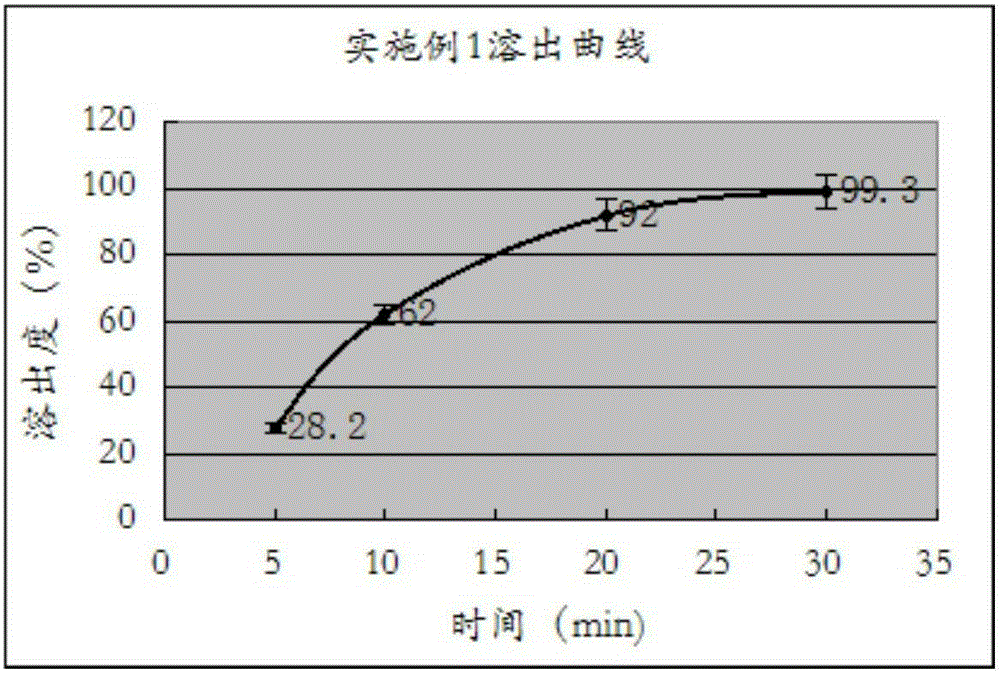
[0023] Measure according to the method under the two appendices of Chinese Pharmacopoeia version in 2010, paddle method, temperature is 37 ℃, and rotating speed is 50 rev / mins, and dissolution medium is in the 0.1M hydrochloric acid solution that contains 0.45% sodium lauryl sulfate in 500ml, uses The ultraviolet detector detects under the wavelength 233nm, obtains the dissolution measurement result in the embodiment one, as shown in specific reference table 1, and forms as follows figure 1 The dissolution profile is shown.
[0024] time (min)
[0025] Table 1: Example 1 dissolution test results
Embodiment 2
[0027]
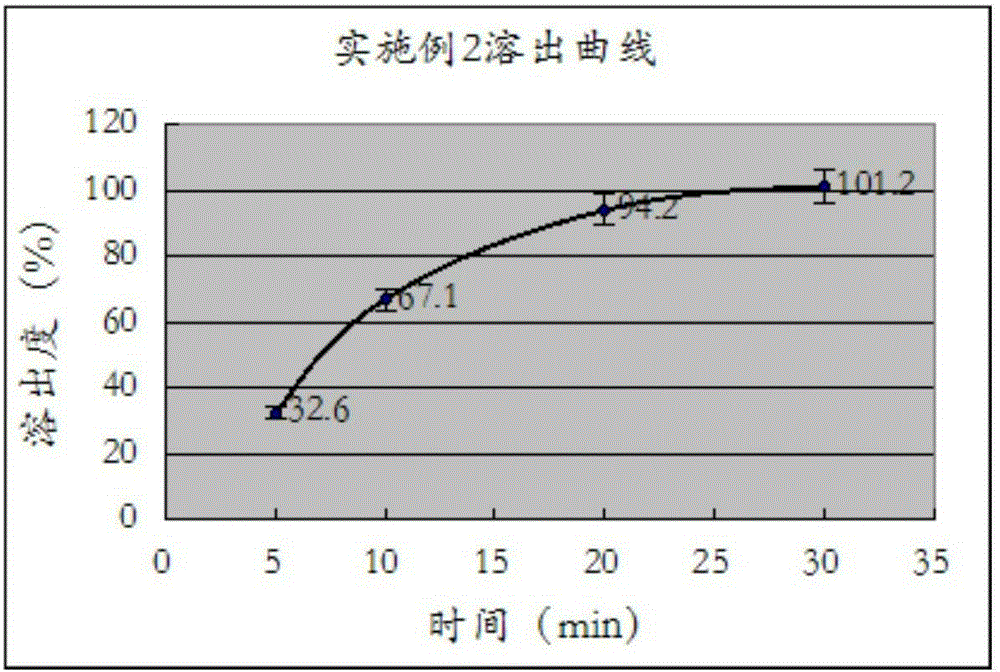
[0028] Measure according to the method under the two appendices of Chinese Pharmacopoeia version in 2010, paddle method, temperature is 37 ℃, and rotating speed is 50 rev / mins, and dissolution medium is in the 0.1M hydrochloric acid solution that contains 0.45% sodium lauryl sulfate in 500ml, uses The ultraviolet detector detects under the wavelength 233nm, obtains the dissolution measurement result in the embodiment one, as shown in the specific reference table 2, and forms as follows figure 2 The dissolution profile is shown.
[0029] time (min)
[0030] Table 2: Dissolution test result of embodiment two
Embodiment 3
[0032]
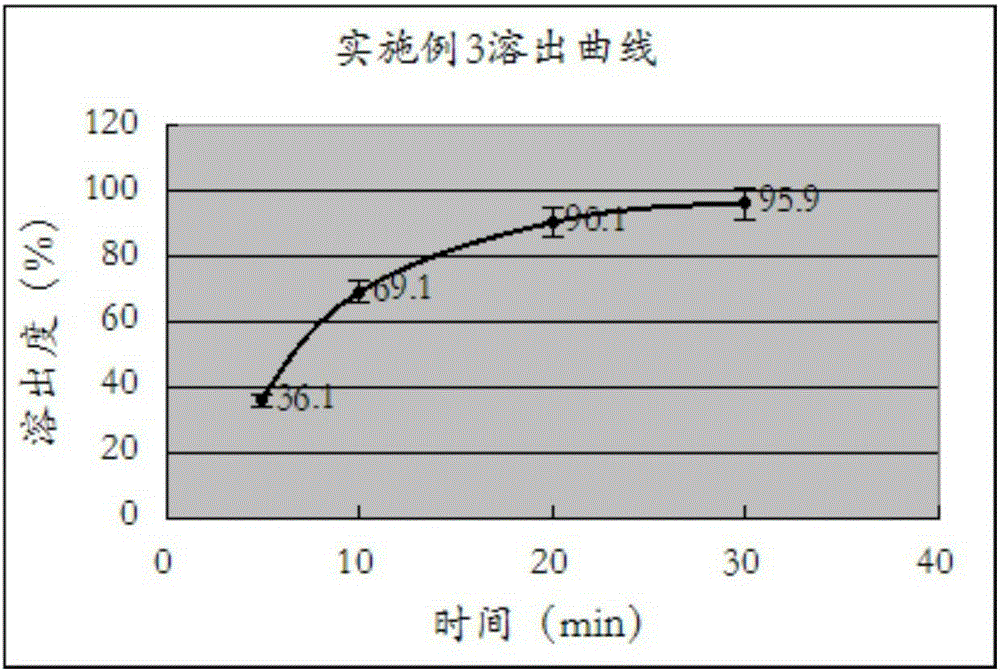
[0033] Measure according to the method under the two appendices of Chinese Pharmacopoeia version in 2010, paddle method, temperature is 37 ℃, and rotating speed is 50 rev / mins, and dissolution medium is in the 0.1M hydrochloric acid solution that contains 0.45% sodium lauryl sulfate in 500ml, uses The ultraviolet detector detects under the wavelength 233nm, obtains the dissolution measurement result in the embodiment one, as shown in the specific reference table 3, and forms as follows image 3 The dissolution profile is shown.
[0034] time (min)
[0035] Table 3: Example 3 dissolution test results
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com