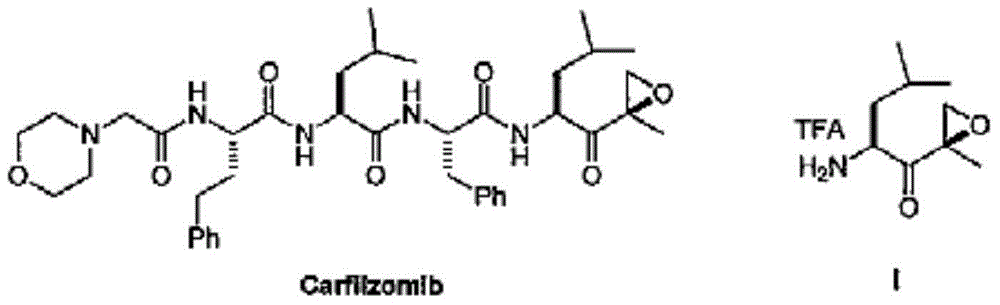
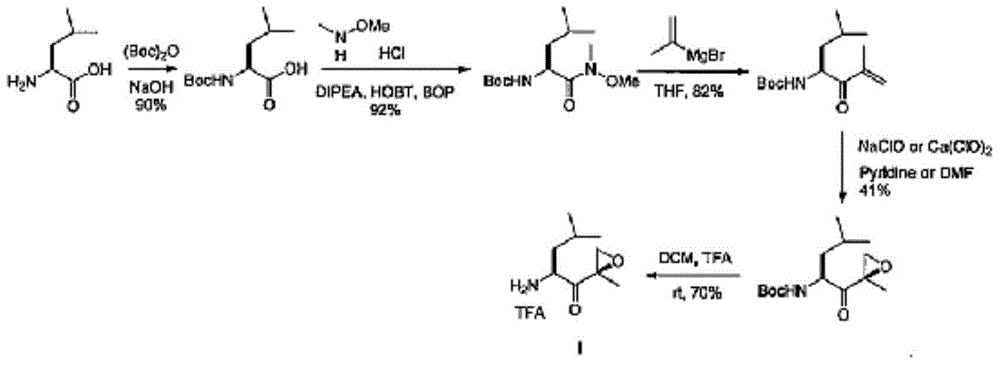
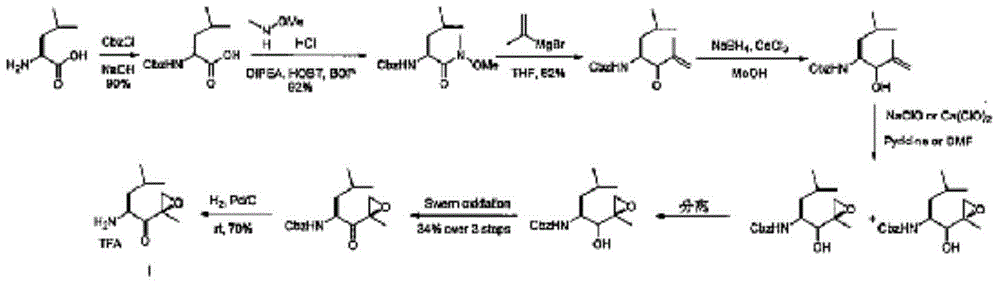
Preparation method of carfilzomib intermediate and intermediate chemical compounds of carfilzomib
A technology of carfilzomib and compounds, which is applied in the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc., can solve problems such as industrialization difficulties, and achieve the effects of good yield, cost reduction, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (1) Preparation of N,N-dibenzyl-L-leucine
[0056]
[0057] L-leucine (262g, 2mol) and benzyl bromide (697g, 4.1mol) were added to N,N-dimethylformamide (3000ml), then anhydrous potassium carbonate (552g, 4mol) was added, heated Reflux, react for 9 hours, TLC detects that the raw material spots disappear, concentrate to remove most of N,N-dimethylformamide, add water and ethyl acetate, extract, separate the water layer, adjust the pH to 3 with 1N hydrochloric acid, and then add acetic acid Ethyl ester was extracted, and the organic layer was separated. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain 541.1 g of N,N-dibenzyl-L-leucine with a yield of 87%.
[0058] 1HNMR (400MHz, DMSO-d6): δppm12.48(br,1H),7.34-7.15(m,10H),4.19(s,4H),3.93-3.88(m,1H),1.72-1.65(m,2H ), 1.54-1.49 (m, 1H), 0.89-0.86 (m, 6H). ESI / MS: m / z=312 (M+H)+.
[0059] (2) Preparation of N,N-dibenzyl-L-leucine-N'-methoxy-N'-...
Embodiment 2
[0076] (1) Preparation of N,N-dibenzyl-L-leucine
[0077]
[0078] L-leucine (262g, 2mol) and benzyl chloride (519g, 4.1mol) were added to N,N-dimethylformamide (3000ml), then anhydrous potassium carbonate (552g, 4mol) was added, heated Reflux, react for 9 hours, TLC detects that the raw material spots disappear, concentrate to remove most of N,N-dimethylformamide, add water and ethyl acetate, extract, separate the water layer, adjust the pH to 3 with 1N hydrochloric acid, and then add acetic acid Ethyl ester was extracted, and the organic layer was separated. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain 510 g of N,N-dibenzyl-L-leucine with a yield of 82%.
[0079] 1HNMR (400MHz, DMSO-d6): δppm12.48(br,1H),7.34-7.15(m,10H),4.19(s,4H),3.93-3.88(m,1H),1.72-1.65(m,2H ), 1.54-1.49 (m, 1H), 0.89-0.86 (m, 6H). ESI / MS: m / z=312 (M+H)+.
[0080] (2) Preparation of N,N-dibenzyl-L-leucine-N'-methoxy-N'-f...
Embodiment 3
[0095] (1) Preparation of N,N-dibenzyl-L-leucine
[0096]
[0097] L-leucine (262g, 2mol) and benzyl bromide (759.5g, 6mol) were added to N,N-dimethylformamide (3500ml), then anhydrous potassium carbonate (690g, 5mol) was added, heated Reflux, react for 10 hours, TLC detects that the raw material spots disappear, concentrate to remove most of N,N-dimethylformamide, add water and ethyl acetate, extract, separate the water layer, adjust the pH to 3 with 1N hydrochloric acid, and then add acetic acid Ethyl ester was extracted, and the organic layer was separated. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated to obtain 578.4 g of N,N-dibenzyl-L-leucine with a yield of 93%.
[0098] 1HNMR (400MHz, DMSO-d6): δppm12.48(br,1H),7.34-7.15(m,10H),4.19(s,4H),3.93-3.88(m,1H),1.72-1.65(m,2H ), 1.54-1.49 (m, 1H), 0.89-0.86 (m, 6H). ESI / MS: m / z=312 (M+H)+.
[0099] (2) Preparation of N,N-dibenzyl-L-leucine-N'-methoxy-N'...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com