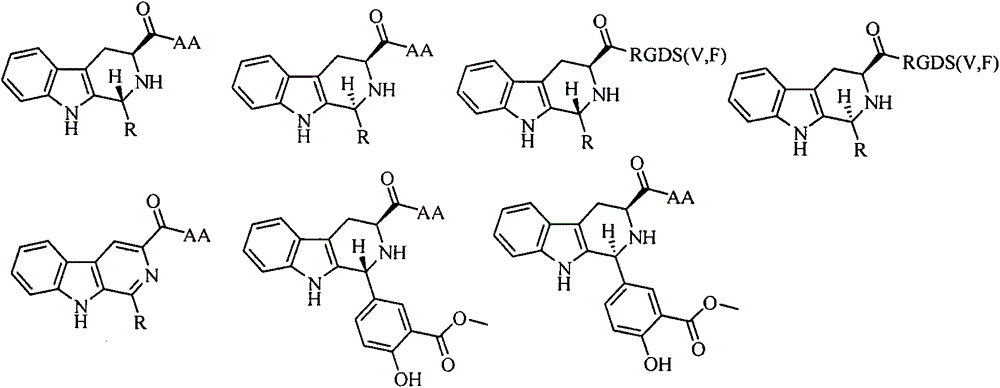
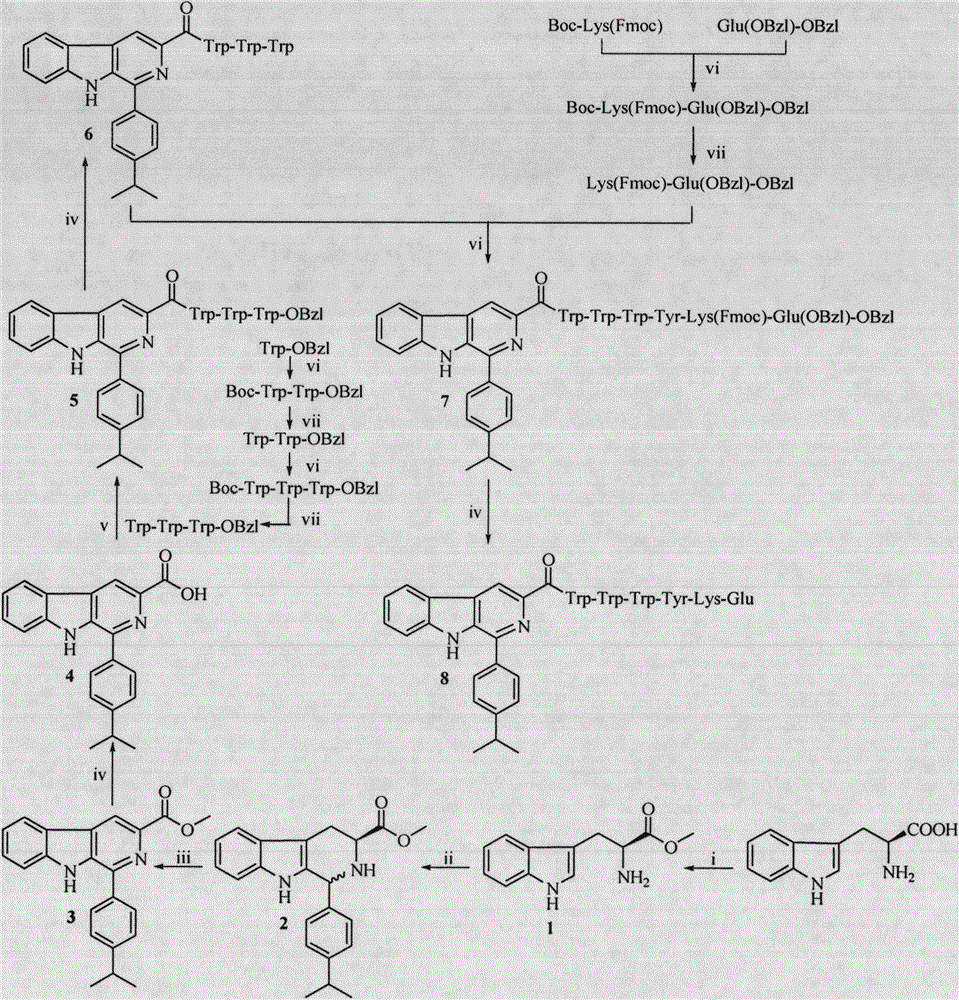
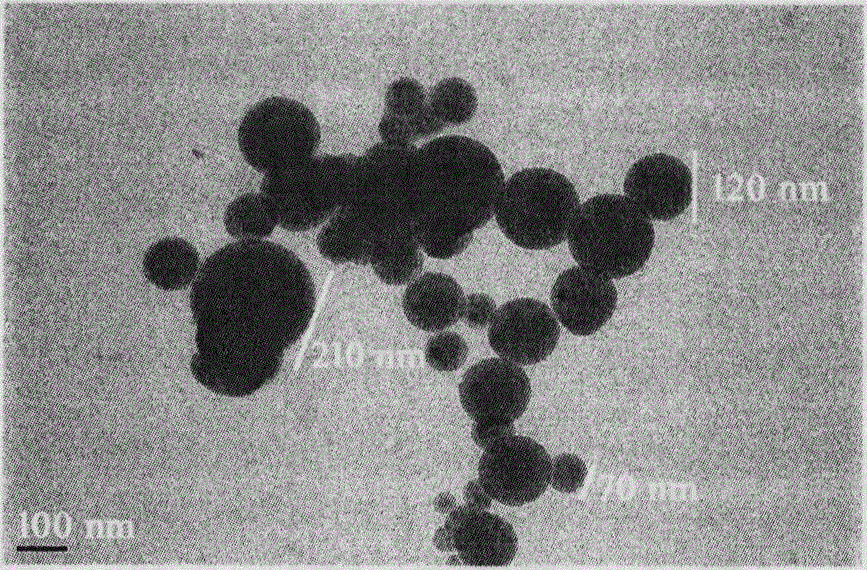
Trp-Trp-Trp pentapeptide modified beta-carboline, preparation therefor, nanostructure, activity and application thereof
A carboline and reaction technology, which is applied in the field of anti-tumor effect in vitro and in vivo, and the preparation of anti-tumor drugs, can solve the problems of not getting anti-tumor and anti-tumor metastatic compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 prepares L-tryptophan methyl ester hydrochloride (1)
[0030] Measure 100mL of methanol into a 250mL eggplant bottle, add 25.5mL of SOCl to the eggplant bottle under ice bath 2 , Plug the drying tube, and after stirring for 30min, add 20.400g (100mmol) of L-tryptophan which has been weighed. Stir at room temperature for 48 h, monitor the reaction by TLC, terminate the reaction when the raw material point disappears, concentrate under reduced pressure to remove the solvent, add 30 mL of methanol, mix, concentrate again under reduced pressure to remove the solvent, and repeat the operation twice. Finally, 50 mL of diethyl ether was added, suspended for 30 min, filtered, and the filter cake was dried and weighed to obtain 22.98 g (90.2%) of the target compound as a gray powder. ESI-MS(m / e): 219[M+H] + .
Embodiment 2
[0031] Example 2 Preparation of (3S)-1-(4-isopropylphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester (2)
[0032] Measure 100mL of distilled water into a 250mL eggplant bottle, add 10mL of trifluoroacetic acid under ice bath, stir for 5min, add 20.320g (80mmol) of L-tryptophan methyl ester hydrochloride, stir until dissolved, add 10.672g (88mmol) of iso Propylbenzaldehyde was stirred at room temperature for 24 hours, TLC showed that the raw material point disappeared, and the reaction was terminated. The reactant was filtered under reduced pressure, the filter cake was dissolved with ethyl acetate, and saturated NaHCO 3 The aqueous solution was adjusted to pH 8. The ethyl acetate layer was extracted and washed 3 times with saturated NaCl aqueous solution, and then washed with 5% KHSO 4 The aqueous solution was extracted and washed once, and the ethyl acetate layer was left to stand, and a colorless solid was precipitated. After drying, it was recrystalliz...
Embodiment 3
[0033] Example 3 Preparation of 1-(4-isopropylphenyl)-β-carboline-3-carboxylic acid methyl ester (3)
[0034]Weigh 13.92g (40mmol) (3S)-1-(4-isopropylphenyl)-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid methyl ester and 5.772g (52mmol) Add selenium dioxide into a 250mL eggplant bottle, add 100mL dioxane to dissolve, place in an oil bath and heat to 75°C, react for 10h, and stop the reaction after TLC monitors that the raw material point disappears. The reactant was cooled to room temperature, filtered under reduced pressure, and the filtrate was concentrated to dryness under reduced pressure to obtain 12.384 g (95%) of the title compound as a yellow solid. ESI-MS(m / e): 345.2[M+H] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com