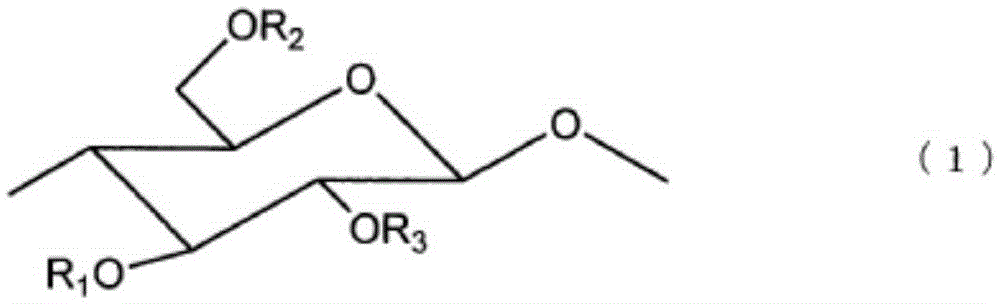
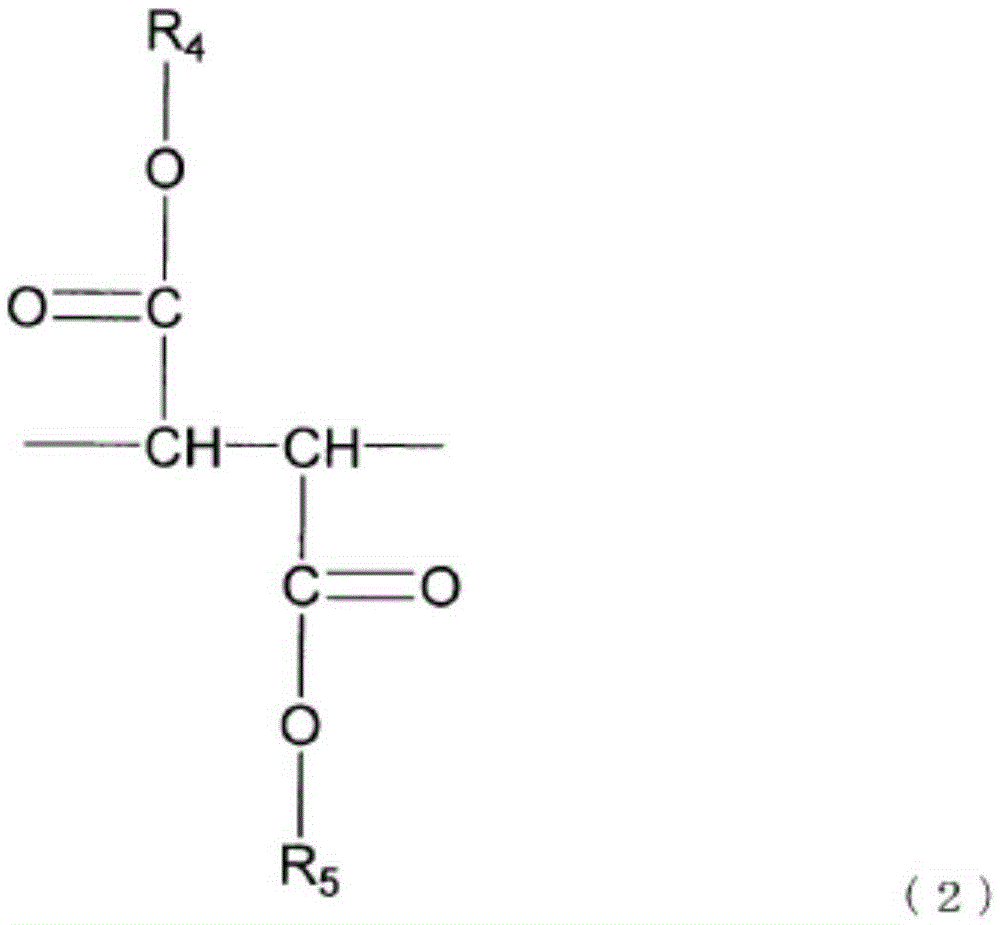
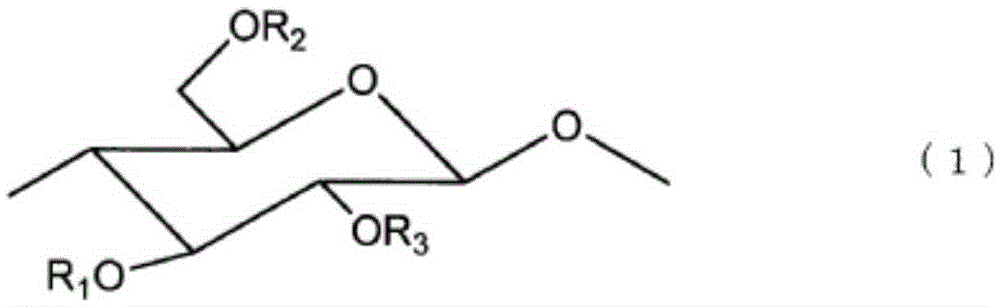
Resin composition, optical compensation film employing same, and production method for same
A technology of resin composition and optical compensation film, which is applied in optics, optical components, nonlinear optics, etc., and can solve the problems that retardation plates cannot meet Re and Nz
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0118] Hereinafter, the present invention will be described with reference to examples, but the present invention is not limited to these examples.
[0119] In addition, various physical properties shown in the Examples were measured by the following methods.
[0120]
[0121] The structural analysis of the polymer was carried out by proton nuclear magnetic resonance emission ( 1 H-NMR) spectral analysis obtained.
[0122]
[0123] Use a gel permeation chromatography (GPC) device (manufactured by Tosoh, trade name: C0-8011 (installed column GMH HR -H)) was measured at 40° C. using tetrahydrofuran or dimethylformamide as a solvent, and calculated in terms of standard polystyrene.
[0124]
[0125] Regarding the measurement of the light transmittance and haze of the produced film, the light transmittance was measured in accordance with JIS K7361-1 (1997 edition), and the haze was measured using a haze meter (manufactured by Nippon Denshoku Industries, trade name: NDH2000...
Synthetic example 1
[0130] Synthesis example 1 (synthesis of diethyl fumarate polymer)
[0131] 50 g of diethyl fumarate and 0.45 g of tert-butyl peroxypivalate as a polymerization initiator were placed in a glass ampoule with a capacity of 75 mL, and after nitrogen substitution and pressure release were repeated, the mixture was sealed under reduced pressure. This ampoule was placed in a thermostat at 50° C., and held for 72 hours to perform radical polymerization. After the polymerization reaction was completed, the polymer was taken out from the ampoule and dissolved in 200 g of tetrahydrofuran. This polymer solution was dropped into 4 L of hexane to precipitate, and then vacuum-dried at 80° C. for 10 hours to obtain 26 g of a diethyl fumarate polymer. The number average molecular weight of the obtained polymer was 25,000.
Synthetic example 2
[0132] Synthesis example 2 (synthesis of diethyl fumarate / monoethyl fumarate copolymer)
[0133] Put 48 g of diethyl fumarate, 2.1 g of monoethyl fumarate, and 0.45 g of tert-butyl peroxypivalate as a polymerization initiator in a glass ampoule with a capacity of 75 mL, and repeat nitrogen substitution and pressure release. , Fusion-sealed under reduced pressure. This ampoule was placed in a thermostat at 50° C., and held for 72 hours to perform radical polymerization. After the polymerization reaction was completed, the polymer was taken out from the ampoule and dissolved in 200 g of tetrahydrofuran. This polymer solution was dropped into 4 L of hexane to precipitate, and then vacuum-dried at 80° C. for 10 hours to obtain 22 g of a diethyl fumarate / monoethyl fumarate copolymer. The obtained polymer had a number average molecular weight of 23,000, 95 mol% of diethyl fumarate residue units, and 5 mol% of monoethyl fumarate residue units.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Width | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com