3,4-epoxy-1-butene preparation method
A technology of butene and epoxy, which is applied in the field of preparation of 3,4-epoxy-1-butene, can solve the problems of waste, low conversion rate of raw materials and low product yield, and achieve good technical effect and technical economy Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
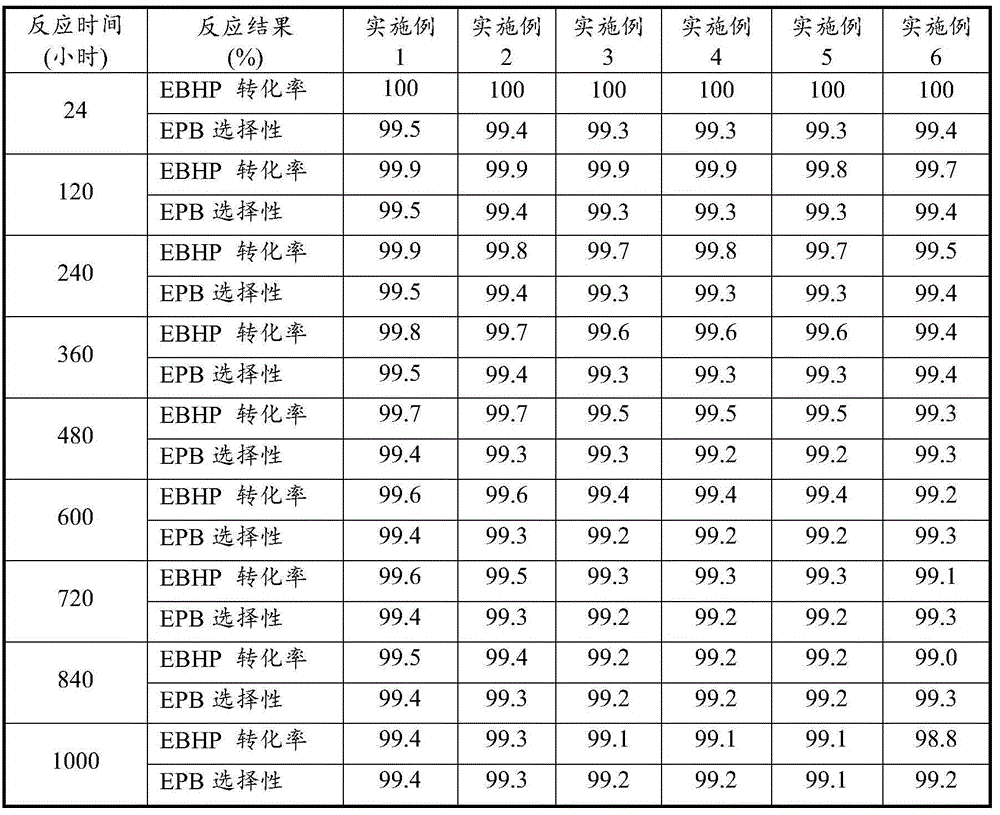
Embodiment 1
[0033] Under the conditions of 160°C, 0.4MPa, and controlling the volume content of tail oxygen to less than 5%, ethylbenzene and air undergo peroxidation reaction to obtain hydrogen peroxide ethylbenzene oxidation solution with a weight concentration of 10%, which is obtained by vacuum concentration. Concentration of 30% ethylbenzene hydrogen peroxide (EBHP) oxidation solution.
[0034] The above-mentioned ethylbenzene hydrogen peroxide oxidation solution with a weight concentration of 30% is carried out in a fixed bed reactor with 1,3-butadiene in the presence of a Ti-HMS catalyst (the weight percentage of titanium is 1.6%) Epoxidation produces 3,4-epoxy-1-butene, α-methylbenzyl alcohol and a small amount of acetophenone. Wherein 1,3-butadiene / EBHP=4 (molar ratio), the gravimetric space velocity of EBHP=3 hours -1 , the reaction temperature is 105°C, and the reaction pressure is 3.5MPa.
[0035] Pass the reaction mixture into a 1,3-butadiene recovery tower to recover exces...
Embodiment 2
[0037] Under the conditions of 150°C, 0.3MPa, and controlling the volume content of tail oxygen to less than 5%, ethylbenzene and air undergo peroxidation reaction to obtain hydrogen peroxide ethylbenzene oxidation solution with a weight concentration of 10%, which is obtained by vacuum concentration. Concentration of 28% ethylbenzene hydroperoxide (EBHP) oxidation solution.
[0038] The above weight concentration is 28% ethylbenzene hydrogen peroxide oxidation solution in the presence of Ti-MCM41 catalyst (the weight percentage of titanium is 2.0%), and 1,3-butadiene is cyclized in a fixed bed reactor The oxidation reaction generates 3,4-epoxy-1-butene, α-methylbenzyl alcohol and a small amount of acetophenone. Wherein 1,3-butadiene / EBHP=4 (molar ratio), the gravimetric space velocity of EBHP=3 hours -1 , the reaction temperature is 105°C, and the reaction pressure is 3.5MPa.
[0039] Pass the reaction mixture into the butadiene recovery tower to recover excess 1,3-butadien...
Embodiment 3
[0041] Under the conditions of 155°C, 0.35MPa, and controlling the volume content of tail oxygen to less than 5%, ethylbenzene and air undergo peroxidation reaction to obtain hydrogen peroxide ethylbenzene oxidation solution with a weight concentration of 10%, which is obtained by vacuum concentration. Concentration of 30% ethylbenzene hydrogen peroxide (EBHP) oxidation solution.
[0042] The above weight concentration is 30% ethylbenzene hydrogen peroxide oxidation solution in the presence of Ti-TUD-1 catalyst (the weight percentage of titanium is 1.5%), in a fixed bed reactor and 1,3-butadiene The epoxidation reaction generates 3,4-epoxy-1-butene, α-methyl benzyl alcohol and a small amount of acetophenone. Wherein 1,3-butadiene / EBHP=4 (molar ratio), the gravimetric space velocity of EBHP=3 hours -1 , the reaction temperature is 105°C, and the reaction pressure is 3.5MPa.
[0043] Pass the reaction mixture into the butadiene recovery tower to recover excess 1,3-butadiene fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com