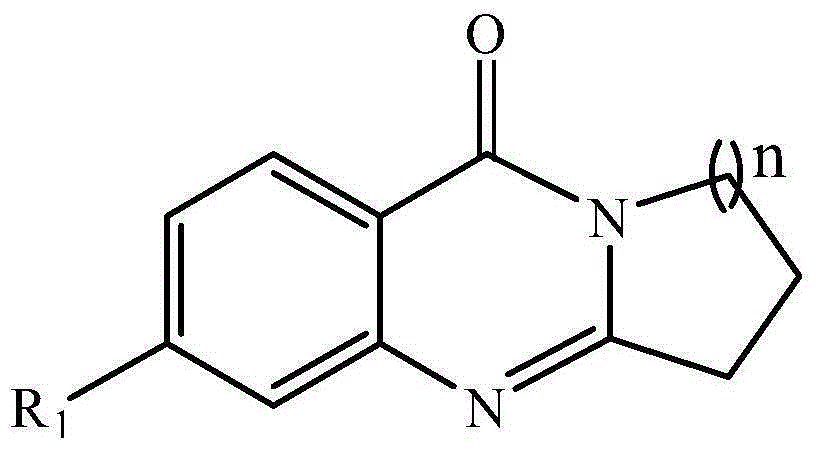
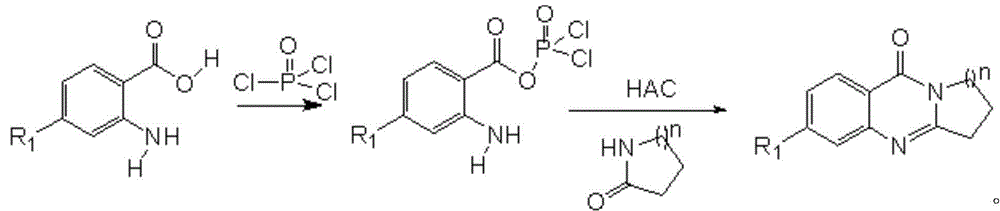
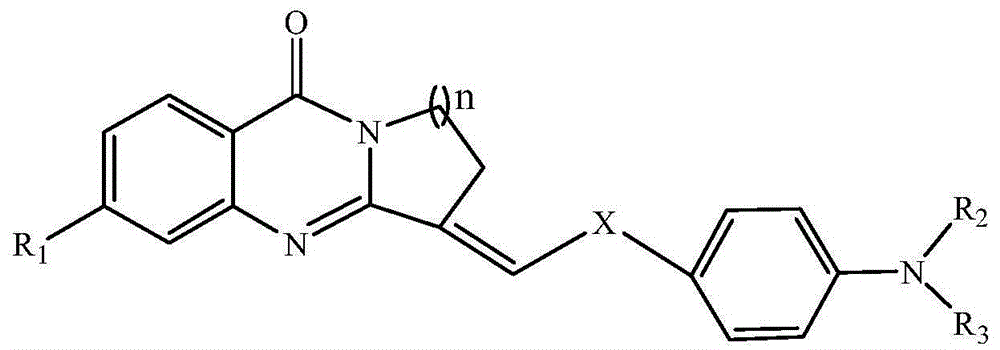
A compound for inhibiting acetylcholinesterase activity, and preparation method and uses thereof
A technology of acetylcholinesterase and compounds, which is applied in the field of chemical synthesis of drugs, can solve problems such as failure to market successfully and inability to reverse the course of the disease, and achieve the effect of low production cost, simple process route, and good acetylcholinesterase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 2. 3-Dihydropyrrolo[2,1-b]quinazolin-9(1H)-one (Ⅰ 1 )
[0039] Add 1.37g (10mmol) anthranilic acid (Ⅲ 1 ), 1.14ml (11mmol) 2-pyrrolidone (Ⅳ 1 ) and 6ml of phosphorus oxychloride, reflux at 100°C for 1 hour under anhydrous conditions, and after natural cooling, pour the reactant into crushed ice, adjust the pH of the system to 8 with potassium bicarbonate, extract three times with 100ml of dichloromethane, reduce The solvent was removed by rotary evaporation. The product was separated by column chromatography using dichloromethane as eluent. Obtain light brown product 1.246g, productive rate 67%, obtained product I 1 The structural formula is as follows:
[0040]
[0041] 2,3-Dihydro-1H-pyrrolo[2,1-b]quinazolin-9-one.
[0042] (CDCl 3 ,500Hz)2.236~2.321(m,2H,CH 2 -CH 2 -CH 2 ),3.140~3.199(m,2H,N=CH 2 -CH 2 -CH 2 ),4.169~4.226(d,2H,CH-CH 2 -N),7.302~7.727(m,3H,Ar-H),8.237~8.280(s,1H,Ar-H).
Embodiment 2
[0044] 6-Chloro-2,3-dihydropyrrolo[2,1-b]quinazolin-9(1H)-one (Ⅰ 2 )
[0045] Add 1.73g (10mmol) 4-chloro-2-aminobenzoic acid (Ⅲ 2 ), 1.14ml (11mmol) 2-pyrrolidone (Ⅳ 1 ) and 6ml of phosphorus oxychloride, reflux at 100°C for 1 hour under anhydrous conditions, and after natural cooling, pour the reactant into crushed ice, adjust the pH of the system to 8 with potassium bicarbonate, extract three times with 100ml of dichloromethane, reduce The solvent was removed by rotary evaporation. DMF / H 2 O mixed solvent recrystallization [69] . 1.652 g of a brown product was obtained, with a yield of 75%. The resulting product I 2 The structural formula is as follows:
[0046]
[0047] 6-Chloro-2,3-dihydro-1H-pyrrolo[2,1-b]quinazolin-9-one
[0048] (CDCl 3 ,500Hz)2.197~2.258(m,2H,N=CH 2 -CH 2 -CH 2 ),3.088~3.120(t,2H,N=CH 2 -CH 2 -CH 2 ),4.109~4.138(t,2H,CH-CH 2 -N), 7.211~7.306(s, H, Ar-H), 7.543~7.547(s, H, Ar-H), 8.103~8.120(s, 1H, Ar-H).
Embodiment 3
[0050] 7,8,9,10-tetrahydroaza[2,1-b]quinazolin-12(6H)-one (Ⅰ 3 )
[0051] Add 1.37g (10mmol) anthranilic acid (Ⅲ 1 ), 1.695g (15mmol) caprolactam (Ⅳ 2 ) and 6ml of phosphorus oxychloride, reflux at 100°C for 1 hour under anhydrous conditions, and after natural cooling, pour the reactant into crushed ice, adjust the pH of the system to 8 with potassium bicarbonate, extract three times with 100ml of dichloromethane, reduce The solvent was removed by rotary evaporation. The product was separated by column chromatography using dichloromethane as eluent. 1.521 g of a yellow product was obtained with a yield of 71%. The resulting product has structural formula I 3 as follows:
[0052]
[0053] 7,8,9,10-Tetrahydro-6H-azepino[2,1-b]quinazolin-12-one
[0054] (CDCl 3 ,500Hz)1.734~1.824(m,6H,CH 2 -CH 2 -CH 2 ),2.984~3.006(t,2H,N=CH 2 -CH 2 -CH 2 ),4.308~4.325(t,2H,CH-CH 2 -N),7.208~7.373(s,H,Ar-H),7.522~7.538(s,H,Ar-H),7.617~7.935(s,H,Ar-H),8.166~8.184(s,1H ,Ar-H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com