A kind of quercetin derivative and its preparation method and application
A derivative, quercetin technology, applied in the field of pharmaceutical preparation, can solve the problems of low bioavailability, large intermolecular attraction, poor water solubility of quercetin, etc., and achieves simple synthesis process requirements and strong acetylcholinesterase inhibitory activity. , the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
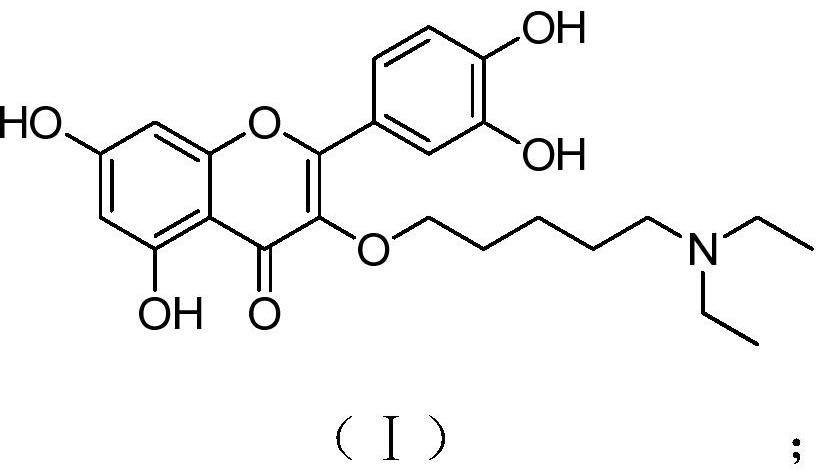
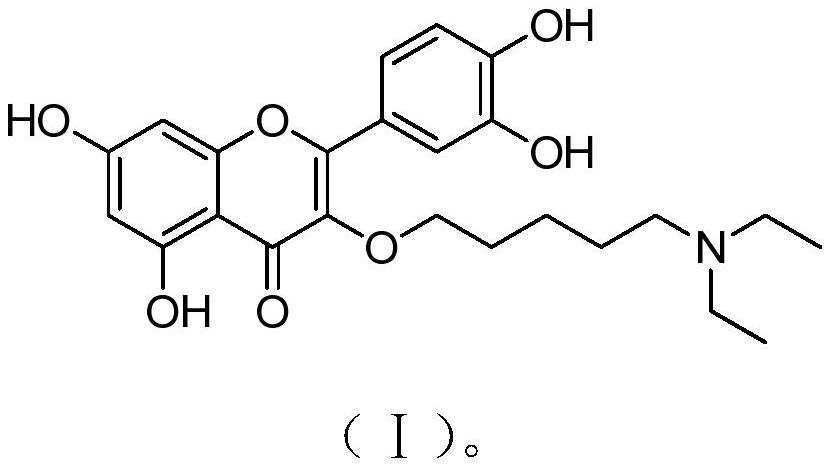
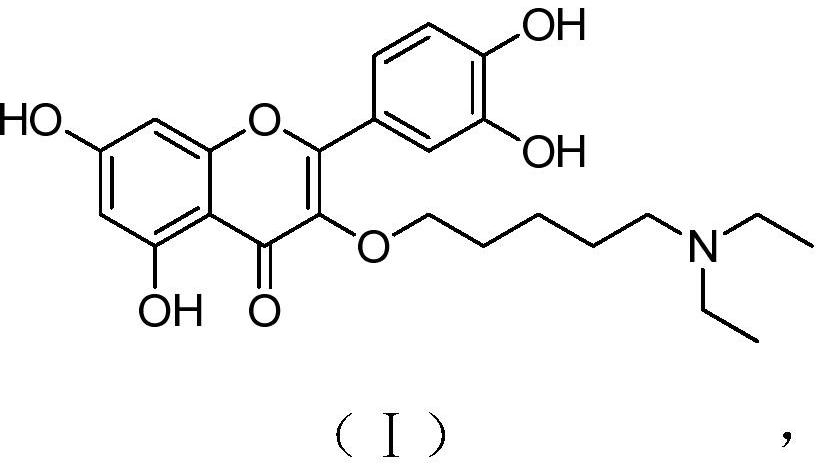
[0026] A kind of quercetin derivative of the present invention, its chemical structural formula has formula (I) shown in:
[0027]
[0028] The chemical name is 2-(3,4-dihydroxyphenyl)-3-(5-N,N-diethylaminopentyloxy)-5,7-dihydroxy-4H-1-benzopyran-4 -ketone.
[0029] The preparation method of quercetin derivative of the present invention comprises the following steps:
[0030] (1) Preparation of 7,3',4'-tribenzyloxyquercetin; dry rutin dehydrated from crystallization water was dissolved in anhydrous DMF, then added anhydrous potassium carbonate and stirred at room temperature for 15 minutes, then Add benzyl bromide and stir at 53°C for 4 hours. After the reaction, use 10% acetic acid to adjust the pH value to 6.5, filter to obtain a precipitate, dissolve the precipitate in absolute ethanol, add concentrated hydrochloric acid, and reflux the mixture at 78°C for 2 hours. hours, cooled to room temperature and filtered to obtain a light yellow solid, which was recrystallized f...
Embodiment 2
[0039] The difference between embodiment 2 and embodiment 1 is:
[0040] The preparation method of quercetin derivative of the present invention comprises the following steps:
[0041] (1) Preparation of 7,3',4'-tribenzyloxyquercetin; dry rutin dehydrated from crystallization water was dissolved in anhydrous DMF, then added anhydrous potassium carbonate and stirred at room temperature for 15 minutes, then Add benzyl bromide and stir at 50°C for 3 hours. After the reaction, use 10% acetic acid to adjust the pH value to 6, filter to obtain a precipitate, dissolve the precipitate in absolute ethanol, add concentrated hydrochloric acid, and reflux the mixture at 78°C for 2.5 hours, cooled to room temperature and filtered to obtain a light yellow solid, which was recrystallized from dichloromethane / methanol to obtain 7,3',4'-tribenzyloxyquercetin. The mass ratio of described rutin, anhydrous potassium carbonate is 34:53.79, and the mass volume ratio of described rutin and benzyl b...
Embodiment 3
[0045] The difference between embodiment 3 and embodiment 1 is:
[0046] The preparation method of quercetin derivative of the present invention comprises the following steps:
[0047] (1) Preparation of 7,3',4'-tribenzyloxyquercetin; dry rutin dehydrated from crystallization water was dissolved in anhydrous DMF, then added anhydrous potassium carbonate and stirred at room temperature for 15 minutes, then Add benzyl bromide and stir at 55°C for 3.5 hours. After the reaction, use 10% acetic acid to adjust the pH value to 6.3, filter to obtain a precipitate, dissolve the precipitate in absolute ethanol, add concentrated hydrochloric acid, and reflux the mixture at 78°C for 2.3 hours, cooled to room temperature and filtered to obtain a light yellow solid, which was recrystallized from dichloromethane / methanol to obtain 7,3',4'-tribenzyloxyquercetin. The volume ratio of the anhydrous DMF, absolute ethanol and concentrated hydrochloric acid is 38:78:15.
[0048] (2) 2-(3,4-dibenz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com