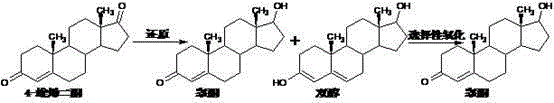
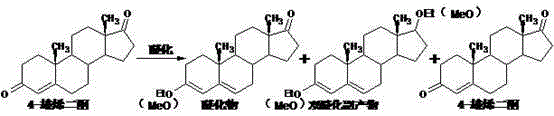
Preparation method of testosterone propionate intermediate 17 beta-hydroxyandrost-4-en-3-one
A technology of hydroxyandrosterone and testosterone propionate, which is applied in the field of preparation of pharmaceutical compound intermediates, can solve the problems of immature technology, limited production scale of fermentation method, and lack of competitive advantages, so as to reduce the refining process of ether compounds and facilitate Realize the effect of industrialization and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Put 400ml of methanol into the reaction bottle, stir to cool down to 0°C, add 40g of acetyl chloride dropwise while stirring to control the temperature to 0-5°C, and continue stirring at 0-5°C for 15 minutes to obtain a spare mixed solution.
[0038] 40 g of 4-androstenedione was put into the above mixed solution to carry out etherification reaction, and the reaction was stirred at 0-5° C. for 5 hours. After the reaction is completed, add the obtained etherification reaction liquid to a solution of 100 g of potassium carbonate dissolved in 3000 ml of water that has been cooled to 0-5°C in advance, and continue stirring for 30 minutes after addition, suction filtration, and water washing to obtain 61.5 etherified wet materials.
[0039] Add 600ml of methanol, 2g of sodium hydroxide dissolved in 10ml of water, and the 61.5 ether compound wet material obtained in the previous step into the reaction flask, and stir for 10 minutes. Cool down, add 6g of potassium borohydride ...
Embodiment 2
[0041] Put 800ml of methanol into the reaction bottle, stir to cool down to 0°C, add 80g of acetyl chloride dropwise while stirring to control the temperature to 0-5°C, and continue stirring at 0-5°C for 30 minutes to obtain a spare mixed solution.
[0042] 40 g of 4-androstenedione was put into the above mixed solution for etherification reaction, and the reaction was stirred at 0-5°C for 4.5 hours. After the reaction is complete, add the obtained etherification reaction liquid to a solution of 110 g of sodium carbonate dissolved in 4000 ml of water that has been pre-cooled to 0-5° C. After the addition, continue stirring for 30 minutes, filter with suction, and wash with water to obtain 61.0 g of etherified wet material.
[0043]Add 600 ml of methanol, 2 g of sodium hydroxide dissolved in 10 ml of water, and the 61.0 ether compound wet material obtained in the previous step into the reaction flask in turn, and stir for 10 minutes. Cool down, add 6.5g of sodium borohydride in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com