Docetaxel polymer micelle freeze-dried preparation and special solvent composition
A technology of docetaxel and polymer glue, applied in the field of medicine and chemical industry, can solve the problems of short validity period, difficult application and increased substance content of drugs, and achieve the effect of solving the problem of chemical stability and physical stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, the preparation of docetaxel polymer micelle freeze-dried preparation
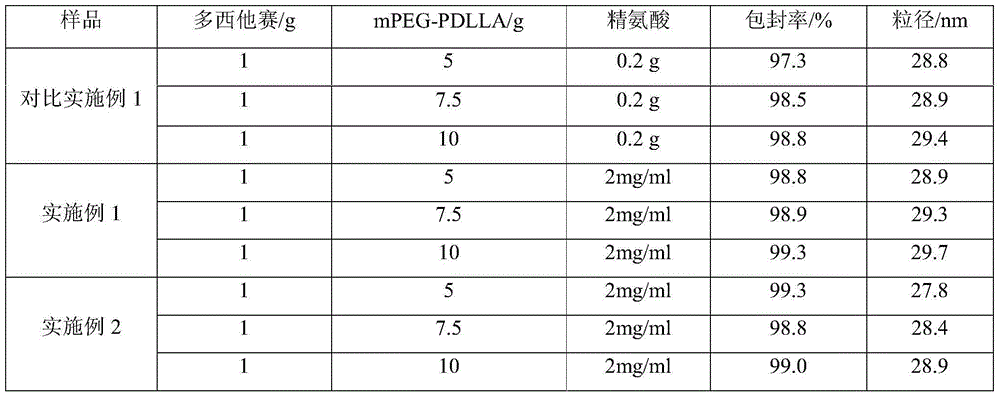
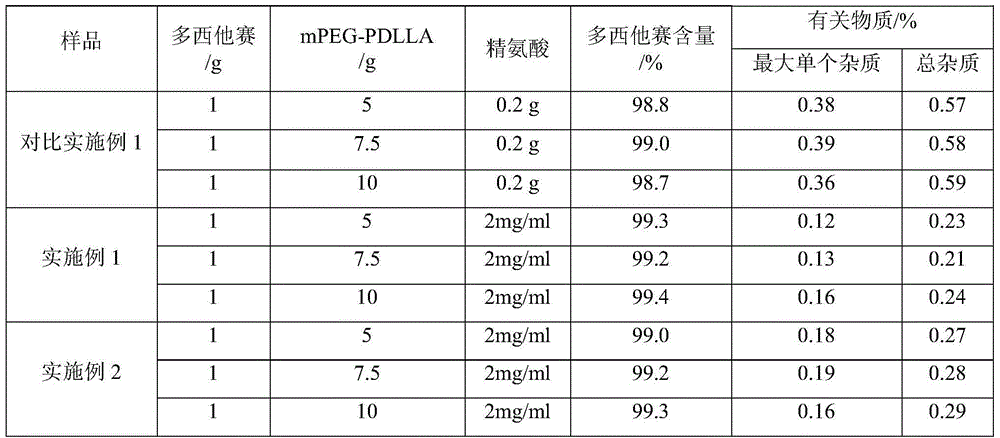
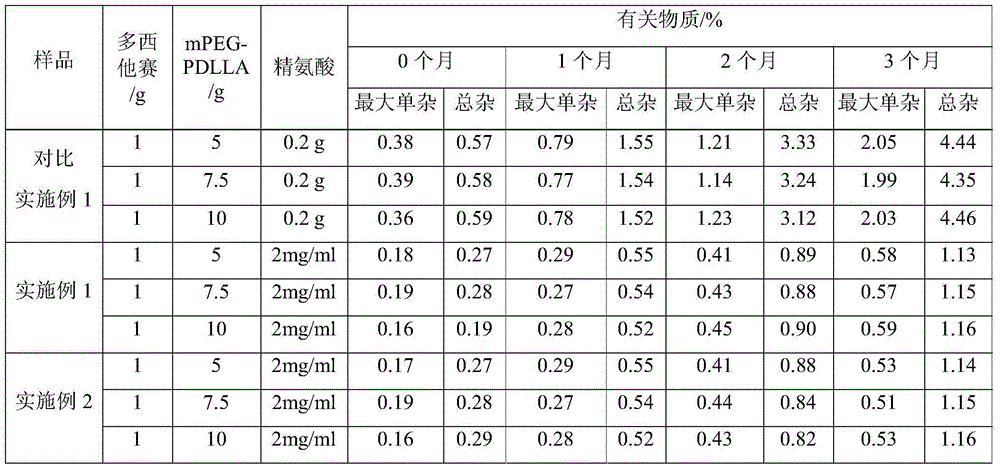
[0020] Weigh 5g polyethylene glycol monomethyl ether-polylactic acid amphiphilic block copolymer (mPEG and PDLLA mass ratio is 40:60) and dissolve it in an appropriate amount of acetonitrile to obtain mPEG-PDLLA acetonitrile solution, and take another 1g docetaxel Docetaxel acetonitrile solution was prepared by dissolving in appropriate amount of acetonitrile, mPEG-PDLLA acetonitrile solution was mixed with docetaxel acetonitrile solution, acetonitrile was removed by rotary evaporation, so that docetaxel and mPEG-PDLLA formed a polymer gel, Then add 100 ml of water for injection, rotate to hydrate to form a polymer micelle solution, add a freeze-drying protective agent, add water to make up the volume, filter with a 0.22 μm microporous membrane, and freeze the filtrate to prepare docetaxel polymer glue Bundle of lyophilized preparations (1:5 mass ratio of docetaxel to mPEG-PDLLA).
[...
Embodiment 2
[0022] Embodiment 2, the preparation of docetaxel polymer micelle freeze-dried preparation
[0023] Weigh 5g of polyethylene glycol monomethyl ether-polylactic acid amphiphilic block copolymer (mPEG and PDLLA mass ratio of 50:50) and dissolve it in an appropriate amount of acetonitrile to obtain mPEG-PDLLA acetonitrile solution, and take another 1g of docetaxel Docetaxel acetonitrile solution was prepared by dissolving in appropriate amount of acetonitrile, mPEG-PDLLA acetonitrile solution was mixed with docetaxel acetonitrile solution, acetonitrile was removed by rotary evaporation, so that docetaxel and mPEG-PDLLA formed a polymer gel, Then add 100 ml of water for injection, rotate to hydrate to form a polymer micelle solution, add a freeze-drying protective agent, add water to make up the volume, filter with a 0.22 μm microporous membrane, and freeze the filtrate to prepare docetaxel polymer glue Bundle of lyophilized preparations (1:5 mass ratio of docetaxel to mPEG-PDLLA)...
Embodiment 3
[0025] Embodiment 3, the preparation of special solvent of docetaxel polymer micelle freeze-dried preparation - arginine aqueous solution
[0026] Dissolve 4g of arginine in 1000ml of water for injection, add 0.2g of activated carbon, make up to 2000ml with water, mix well, let stand for 20 minutes, first filter with a microporous membrane with a pore size of 0.45 μm, and then use a microporous filter with a pore size of 0.22 μm. The microporous membrane filter, the filtrate is divided into vials, plugged, capped, light inspection, packaging, namely the special solvent for docetaxel polymer micelle freeze-dried preparation - arginine aqueous solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com