A photosensitizer with tumor targeting and its preparation method and application
A tumor targeting and photosensitizer technology, applied in the field of biomedicine, can solve the problems of poor water solubility, unstable structure, and no specific targeting of DPP derivative photosensitizers, and achieves excellent water solubility, simple preparation process, excellent Effects of water solubility and tumor targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
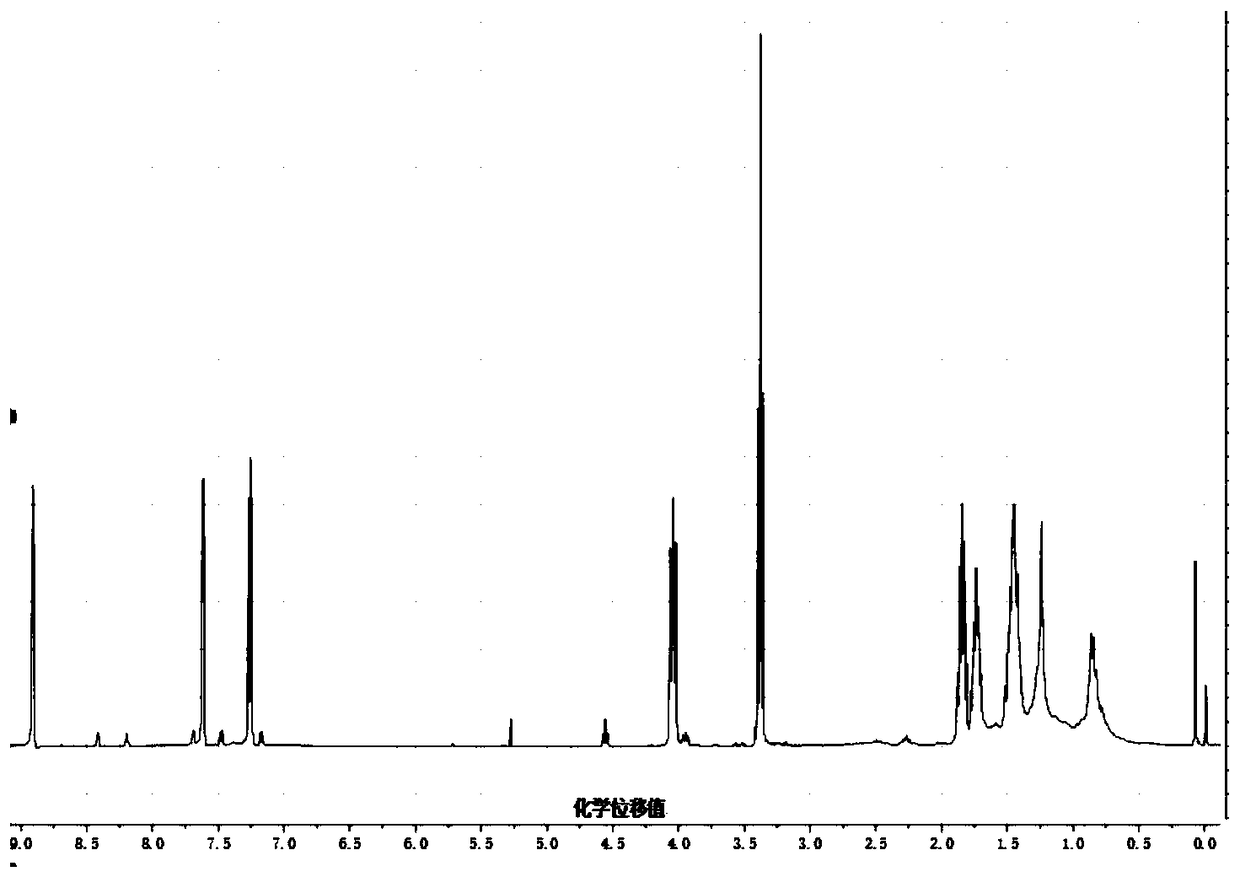
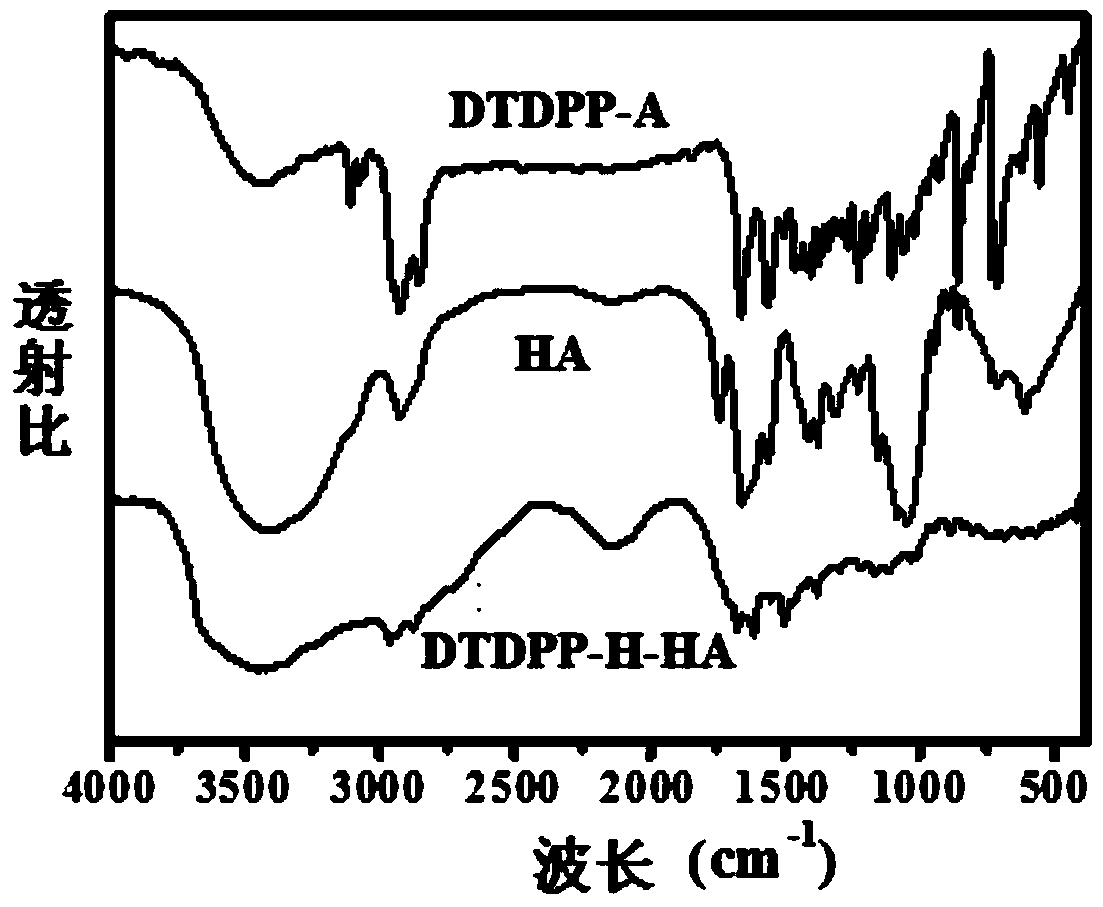
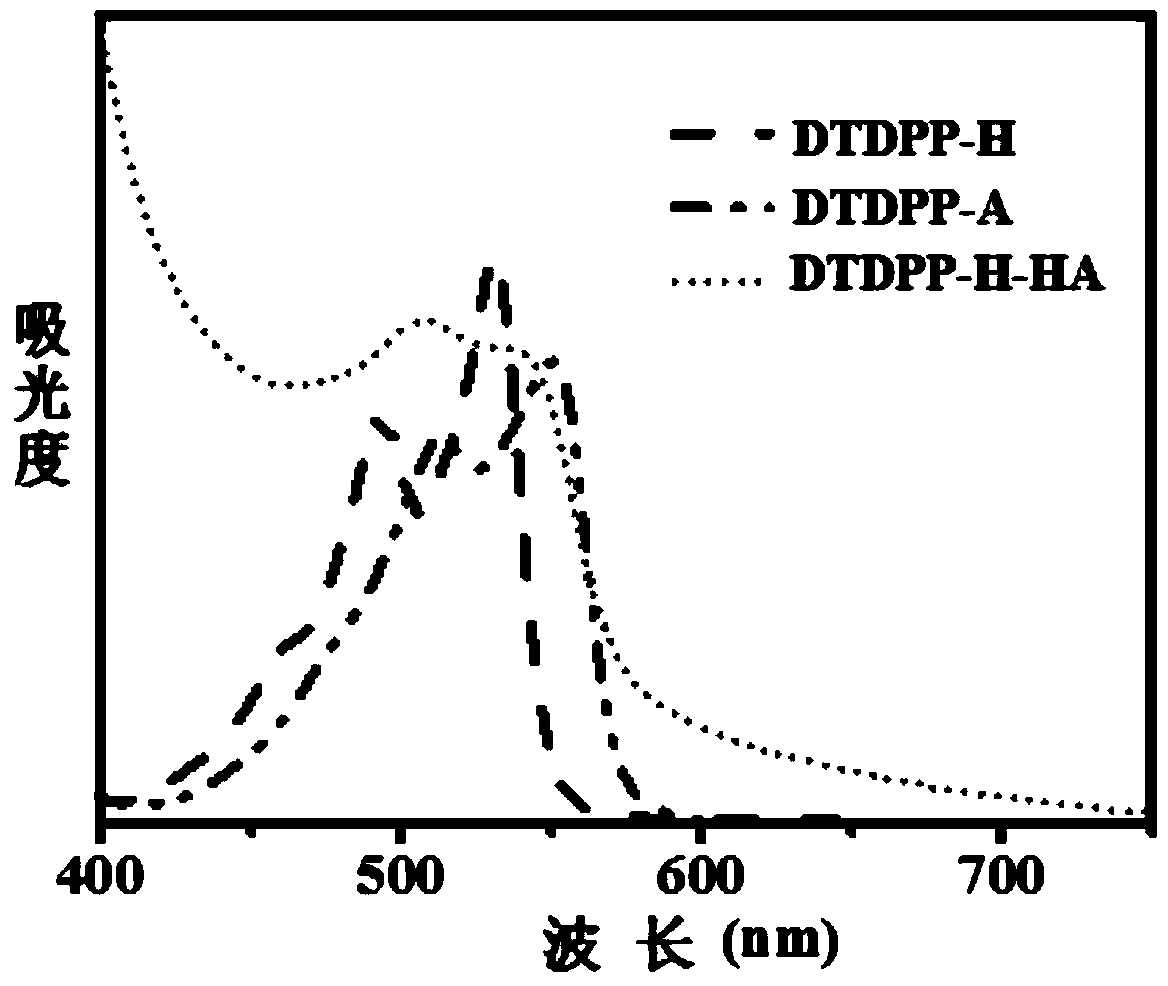
[0038]Synthesis of 3,6-bis(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione covalently bound to hyaluronic acid (DTDPP-H-HA) :
[0039] Under nitrogen protection, starting material 3,6-bis(2-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (1.24g, 5.0mmol), starting material 1 , 6-dibromohexane (6.02g, 12mmol) and raw material anhydrous potassium carbonate (0.52g, 9.28mmol) were added in 25ml DMF, stirred at 55°C for 20h, washed with water, extracted with DCM to remove DMF to obtain crude product, crude The product was separated by silica gel column to obtain the red intermediate DTDPP-A.
[0040] Under the protection of nitrogen, the raw materials tetrabutylammonium hydroxide (2.98g, 29mmol) and sodium hyaluronate (1.16g, 2.8mmol) were added to deionized water, stirred at 40°C for 15h, and freeze-dried to obtain the intermediate HA - .
[0041] Under the protection of nitrogen, the intermediate 3,6-bis(2-thienyl)-2,5-bis(1-bromo-hexane)pyrrolo[3,4-c]pyrrole-1,4 obt...
Embodiment 2
[0047] 3,6-bis(2-bromo-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione covalently bound to hyaluronic acid (DTDPP-Br-HA) Synthesis:
[0048] Under nitrogen protection, raw material 3,6-bis(2-bromo-thienyl)-2,5-dihydropyrrolo[3,4-c]pyrrole-1,4-dione (DTDPP-Br) (2.04g , 5.0mmol), raw material 1,6-dibromohexane (7.525g, 15mmol) and raw material anhydrous potassium carbonate (0.84g, 15mmol) were added to 25ml anhydrous DMF, stirred at 70°C for 15h, washed with water, washed with DCM DMF was removed by extraction to obtain a crude product, which was separated by silica gel chromatography to obtain a red intermediate DTDPP-B.
[0049] Under the protection of nitrogen, the raw material tetrabutylammonium hydroxide (2.98g, 29mmol) and the raw material sodium hyaluronate (1.16g, 2.8mmol) were added to deionized water, stirred at 45°C for 10h, and freeze-dried to obtain the intermediate HA - .
[0050] Under nitrogen protection, the red intermediate 3,6-bis(2-bromo-thienyl)-2,5-b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com