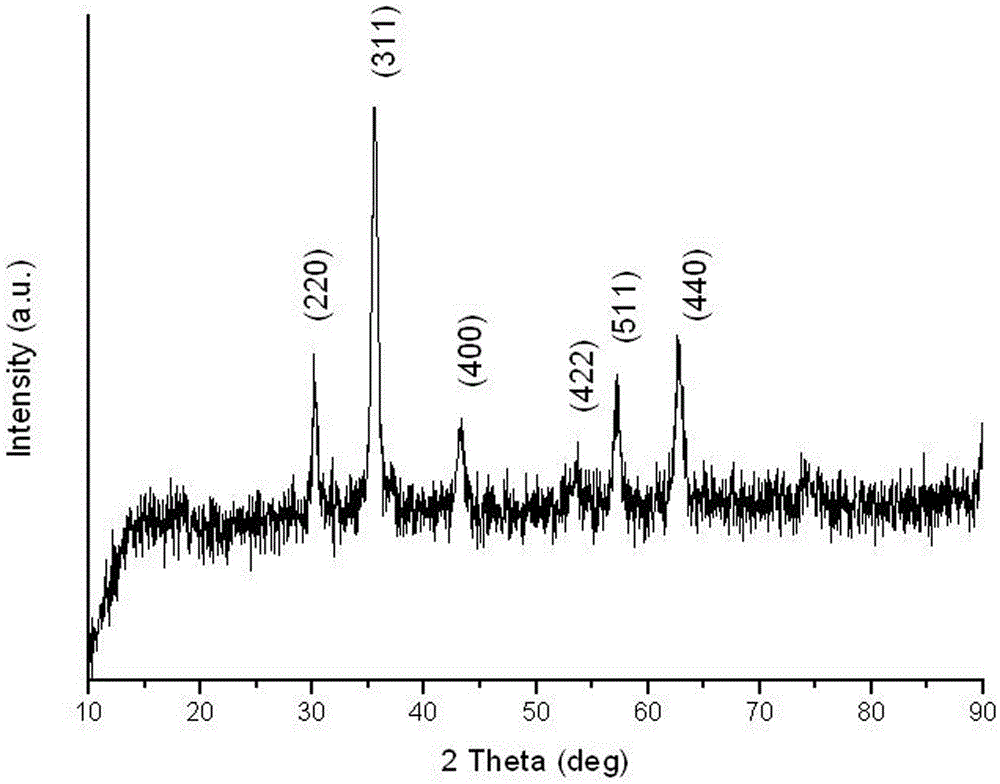
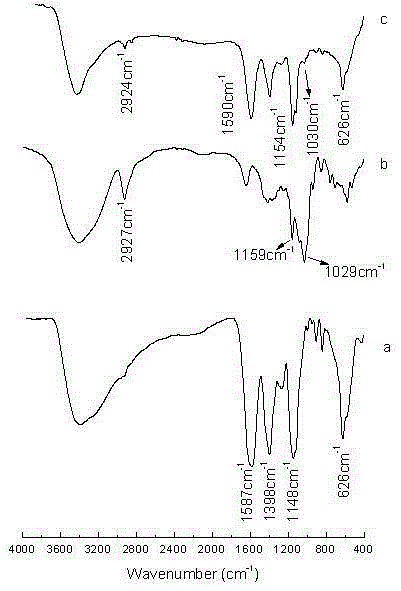
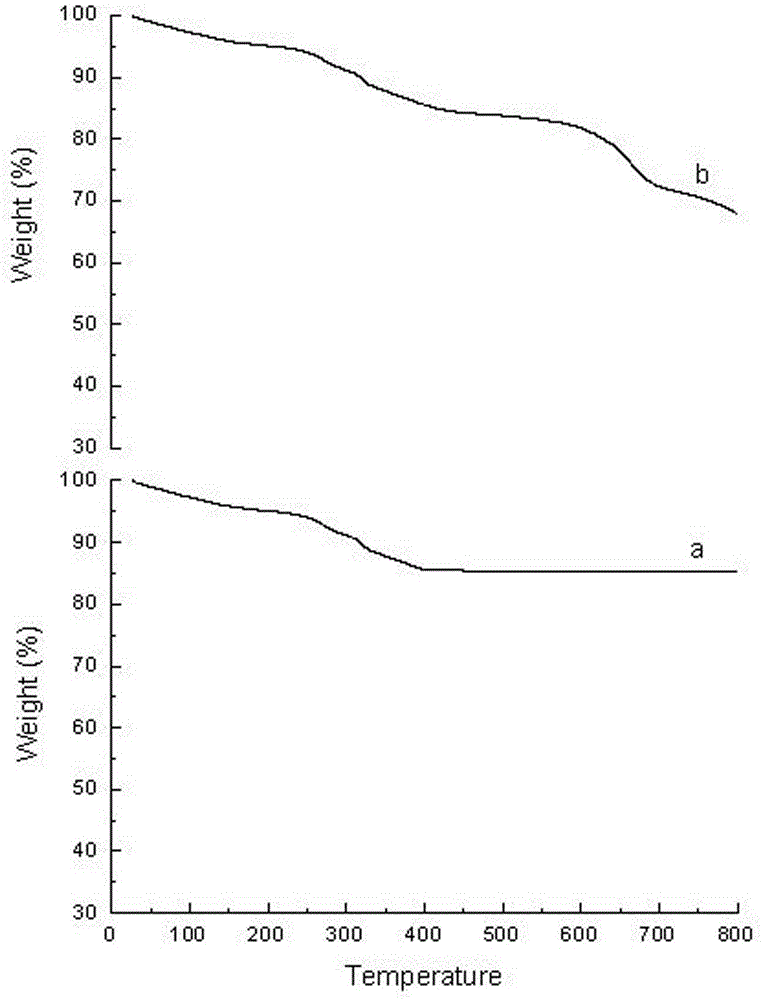
Preparation method and application of Fe3O4@CA-beta-CD nano microspheres
A technology of nano-microspheres and fe3o4ca-, applied in the field of biomedicine, can solve the problems of obvious burst release phenomenon and low drug loading rate in drug release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Fe in this example 3 o 4 The preparation method of CA-β-CD nano microspheres, the steps are as follows:
[0030] (1) Surface-modified ferric oxide nanoparticles Fe of citric acid 3 o 4 Preparation of CA; with FeCl 3 ·6H 2 O, FeSO 4 ·7H 2 O was the iron source, citric acid was used as the stabilizer, and citric acid-modified Fe3O4 was synthesized by ultrasonic-assisted co-precipitation method. 3 o 4 CA nanoparticles, where Fe 3+ with Fe 2+ The molar ratio of the citric acid is 2:1, the consumption of citric acid is 80% of the iron source consumption by mass, the reaction time is 15min, the aging time during coprecipitation method preparation is 30min, and the aging temperature is 90°C;
[0031] (2) Fe 3 o 4 Preparation of CA-β-CD nanospheres: 1gFe 3 o 4 Add CA nanoparticles to 1.5mL distilled water, then add 2g β-cyclodextrin, stir evenly, β is dried in a vacuum oven at 110°C for 3 hours, and the dried samples are washed 3 to 5 times with absolute ethanol a...
Embodiment 2
[0038] Fe in this example 3 o 4 The preparation method of CA-β-CD nano microspheres, the steps are as follows:
[0039] (1) Surface-modified ferric oxide nanoparticles Fe of citric acid 3 o 4 Preparation of CA; with FeCl 3 ·6H 2 O, FeSO 4 ·7H 2 O was the iron source, citric acid was used as the stabilizer, and citric acid-modified Fe3O4 was synthesized by ultrasonic-assisted co-precipitation method. 3 o 4 CA nanoparticles, where Fe 3+ with Fe 2+ The molar ratio of the citric acid is 2:1, the consumption of citric acid is 50% of the iron source consumption by mass, the reaction time is 15min, the aging time during co-precipitation method preparation is 30min, and the aging temperature is 90°C;
[0040] (2) Fe 3 o 4 Preparation of CA-β-CD nanospheres: Fe 3 o 4 Add CA nanoparticles into distilled water, 1gFe 3 o 4 CA nanoparticles need 1mL of distilled water, then add 0.8g of β-cyclodextrin, stir evenly, and dry in a vacuum oven at 115°C for 2.5h. Fe is separated...
Embodiment 3
[0042] Fe in this example 3 o 4 The preparation method of CA-β-CD nano microspheres, the steps are as follows:
[0043] (1) Surface-modified ferric oxide nanoparticles Fe of citric acid 3 o 4 Preparation of CA; with FeCl 3 ·6H 2 O, FeSO 4 ·7H 2 O was the iron source, citric acid was used as the stabilizer, and citric acid-modified Fe3O4 was synthesized by ultrasonic-assisted co-precipitation method. 3 o 4 CA nanoparticles, where Fe 3+ with Fe 2+ The molar ratio of the citric acid is 2:1, the consumption of citric acid is 100% of the iron source consumption by mass, the reaction time is 15min, the aging time during coprecipitation method preparation is 30min, and the aging temperature is 90°C;
[0044] (2) Fe 3 o 4 Preparation of CA-β-CD nanospheres: 1gFe 3 o 4 Add CA nanoparticles to 2 mL of distilled water, then add 1.6 g of β-cyclodextrin, stir evenly, and dry in a vacuum oven at 105 °C for 3.5 hours. The dried samples are washed with absolute ethanol and deoxy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com