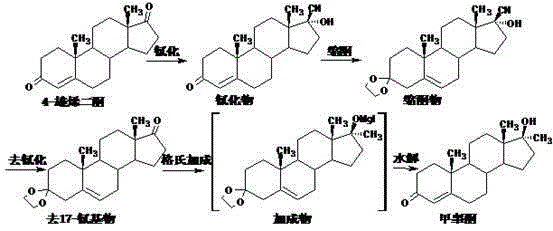
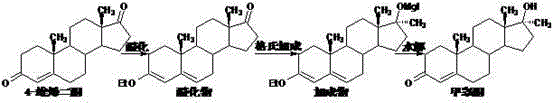
Preparation method for methyltestosterone
A methyltestosterone and ketone-based technology, applied in the field of chemical pharmacy, can solve the problems that etherification reaction is not easy to control, the purity of intermediate etherification products is not high, etc., so as to reduce the purification process of etherate, ensure operation safety and low production cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Put 400ml of methanol into the reaction bottle, stir to cool down to 0°C, add 40g of acetyl chloride dropwise while stirring to control the temperature to 0-5°C, and continue stirring at 0-5°C for 15 minutes to obtain a spare mixed solution.
[0036] 40 g of 4-androstenedione was put into the above mixed solution to carry out etherification reaction, and the reaction was stirred at 0-5° C. for 5 hours. After the reaction is completed, add the obtained etherification reaction liquid to a solution of 100 g of potassium carbonate dissolved in 3000 ml of water that has been cooled to 0-5°C in advance, and continue stirring for 30 minutes after addition, suction filtration, and water washing to obtain 61.5 etherified wet materials.
[0037] Add 18g of magnesium chips, 400ml of tetrahydrofuran, and 0.1g of iodine pellets into the reaction flask, stir and heat up to 40°C, start feeding in methylene chloride at 40°C to 45°C until the reaction of magnesium chips is complete, and ...
Embodiment 2
[0040] Put 800ml of methanol into the reaction bottle, stir to cool down to 0°C, add 80g of acetyl chloride dropwise while stirring to control the temperature to 0-5°C, and continue stirring at 0-5°C for 30 minutes to obtain a spare mixed solution.
[0041] 40 g of 4-androstenedione was put into the above mixed solution for etherification reaction, and the reaction was stirred at 0-5°C for 4.5 hours. After the reaction is complete, add the obtained etherification reaction liquid to a solution of 110 g of sodium carbonate dissolved in 4000 ml of water that has been pre-cooled to 0-5° C. After the addition, continue stirring for 30 minutes, filter with suction, and wash with water to obtain 61.0 g of etherified wet material.
[0042] Add 18g of magnesium chips, 400ml of tetrahydrofuran, and 0.1g of iodine pellets into the reaction flask, stir and heat up to 40°C, start feeding in methylene chloride at 40°C to 45°C until the reaction of magnesium chips is complete, and continue to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com