HPLC detection method for alpinia-cyperus preparations
A detection method and a well-attached technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of non-testing, etc., and achieve the effects of improving safety, comprehensive information reflection, and accurate and reliable measurement results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The preliminary test of embodiment 1 detection method of the present invention
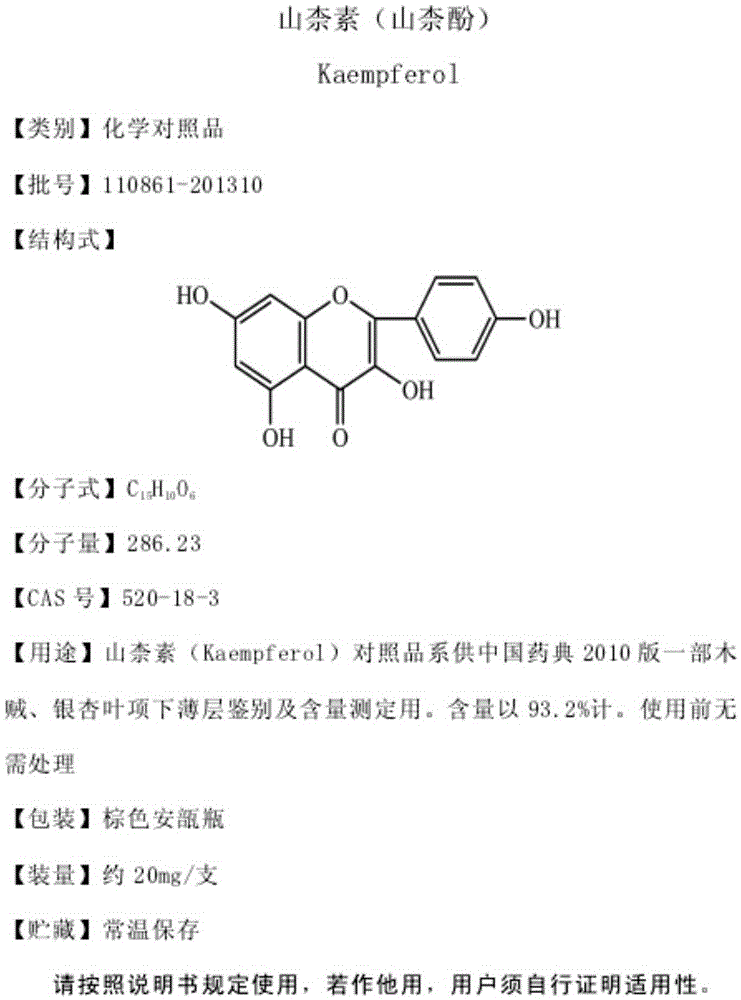
[0069] Since galangin and kaempferol are quite polar, in the preliminary test, the inventor tried a variety of chromatographic conditions including known literature, but it was difficult to separate galangin from kaempferol. For example:
[0070] With reference to the method in the literature "HPLC method is determined the content of galangin and kaempferol in galanga" (Li Zhiyong etc., Chinese Journal of Traditional Chinese Medicine, the 9th phase of the 25th volume in September, 2010, the 1368-1370 page), below The galangin and kaempferol reference substances were determined in the chromatographic conditions:
[0071] Column: Phenomenex C 18 Chromatographic column (4.6mm×250mm, 5μm)
[0072] Detection wavelength: 360nm;
[0073] Mobile phase: methanol-0.4% phosphoric acid (60:40)
[0074] Volume flow: 1.0mL / min;
[0075] Column temperature: 30°C;
[0076] Injection volume: 10μL;
...
Embodiment 2
[0080] Embodiment 2 detection method of the present invention and methodology investigation
[0081] 1. Preparation of reference solution
[0082] Take an appropriate amount of galangin, kaempferol, cyperenone, citronone, and α-cyperone reference substances, accurately weigh them, place them in a 50mL measuring bottle, dissolve them with chromatographic methanol and dilute to the mark to obtain a mixed control stock solution. Precisely measure 1 mL of the above stock solution, put it in a 10 mL measuring bottle, dissolve it with chromatographic methanol and dilute to the mark to obtain the reference substance solution, the mass concentrations are 0.254, 0.032, 0.051, 0.005, 0.021 mg / mL, respectively.
[0083] 2. Preparation of the test solution
[0084] Take about 1g of Liangfu pill powder, weigh it accurately, put it in a stoppered Erlenmeyer flask, accurately add 25mL of methanol, weigh it, ultrasonically treat it (power 250W, frequency 40kHz) for 30min, let it cool, weigh...
Embodiment 3
[0115] The screening test of embodiment 3 detection method of the present invention
[0116] 1. Screening of detection wavelength
[0117] Under the following chromatographic conditions, compare the wavelengths of 235nm, 242nm, 254nm, 266nm, and 280nm.
[0118] Chromatographic column: Phenomenex C18 chromatographic column (4.6mm×250mm, 5μm);
[0119] Mobile phase: methanol-water;
[0120] Volume flow: 1.0mL / min;
[0121] Column temperature: 30°C;
[0122] Gradient elution program:
[0123]
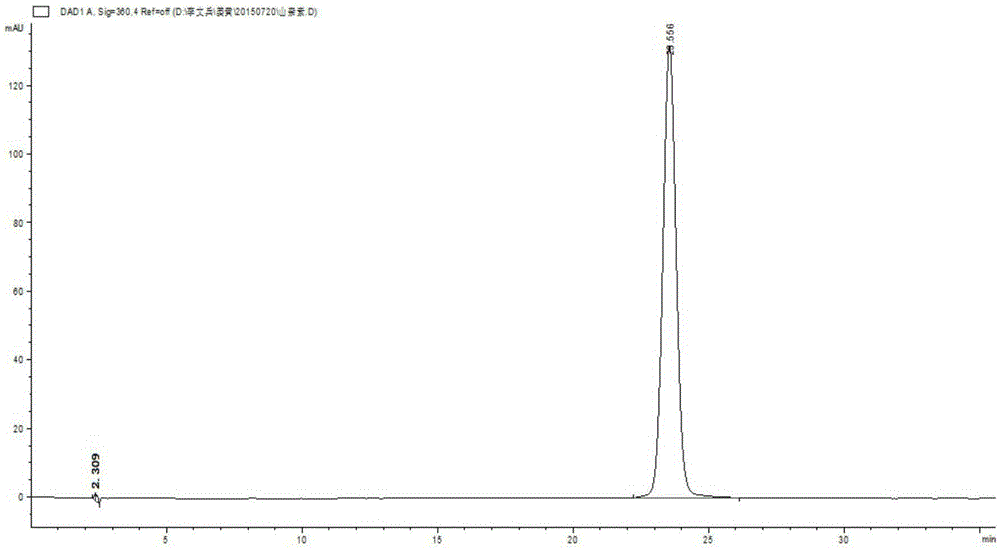
[0124] result Such as Figure 8-12 shown.
[0125] The results show that at 242nm, each component to be tested has a large ultraviolet absorption, so 242nm is selected as the detection wavelength.
[0126] 2. Screening of mobile phase and gradient elution program
[0127] Various mobile phases and gradient elution programs such as methanol-water, methanol-0.2% phosphoric acid, acetonitrile-0.2% phosphoric acid, etc. were investigated, and the conditions were as follows:
[01...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com