Method of producing 3,4-epoxy-1-butylene
A technology of butene and epoxy, applied in the direction of organic chemistry, etc., can solve the problems of low conversion rate of raw materials, wasteful product yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
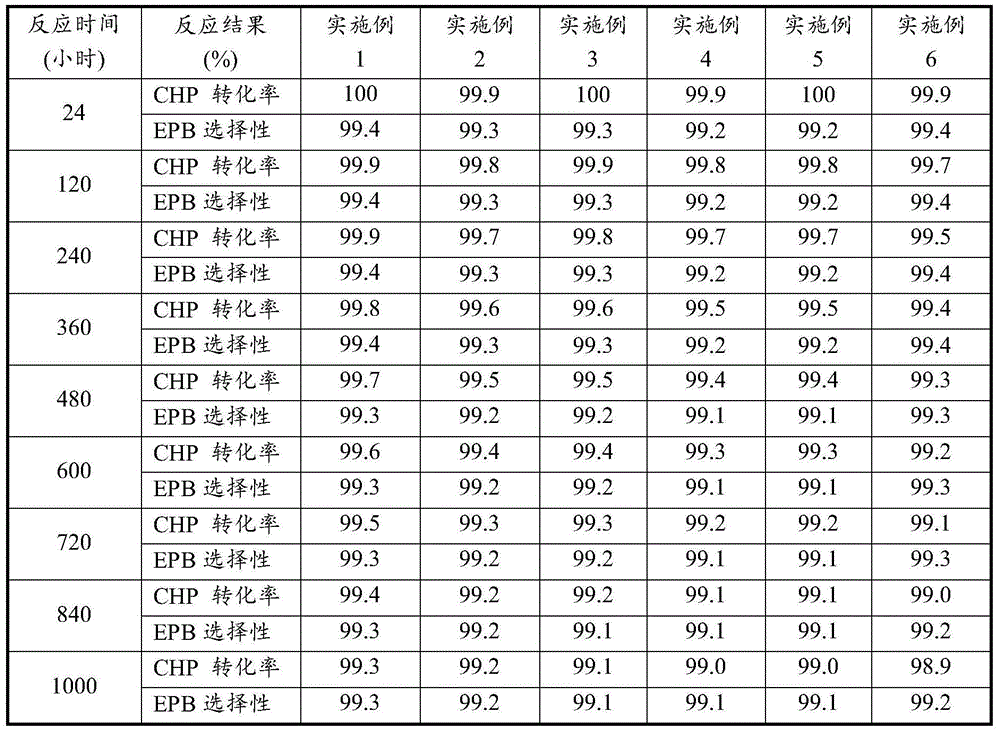
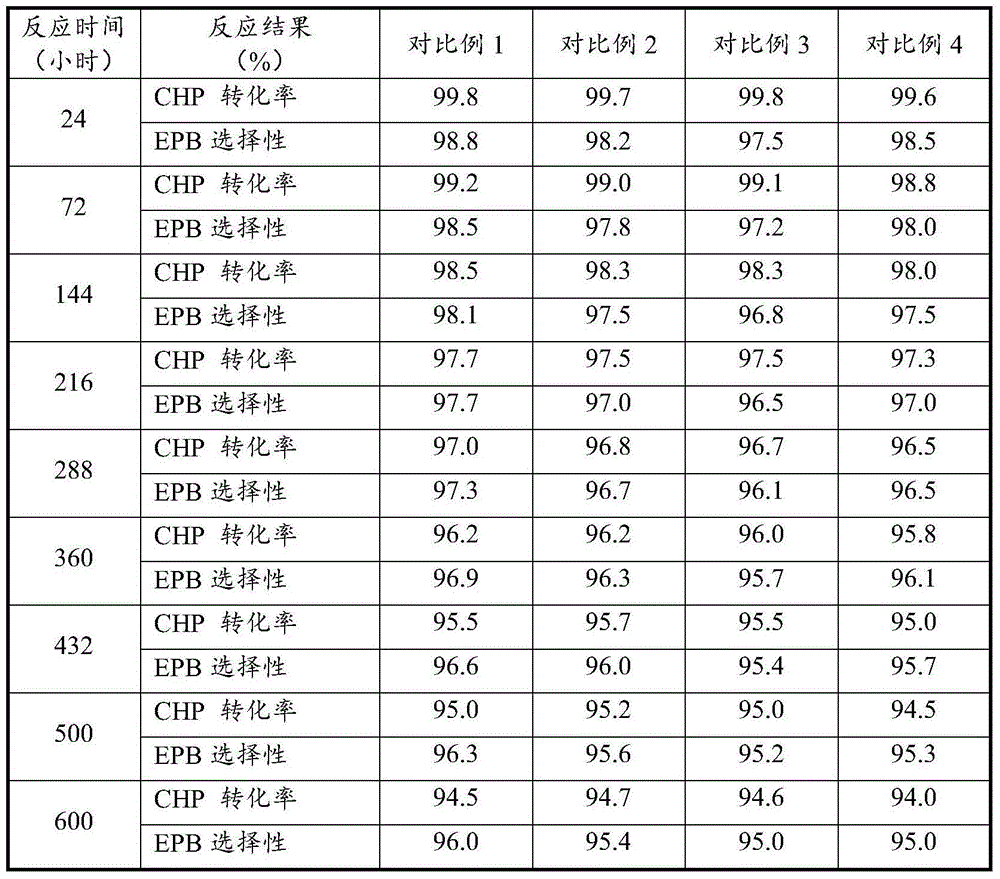
Embodiment 1
[0035] Under the condition of 105 DEG C and 0.4 MPa, and the volume content of tail oxygen is controlled to be less than 5%, the cumene and air are oxidized to obtain cumene hydroperoxide (CHP) oxidation solution with a weight concentration of 20-24%.
[0036] Wash the cumene hydroperoxide oxidation solution with an aqueous solution with a weight content of 2% NaOH, wherein the volume ratio of the oxidation solution to the alkali solution is 3:1, and remove the organic acid therein. Then wash the oxidizing solution with deionized water to remove the residual Na due to alkaline washing + , wherein the volume ratio of oxidizing solution to deionized water is 3:1. According to the needs of the epoxidation reaction, vacuum concentration is carried out to it, the concentration temperature is 75° C., and the cumene hydroperoxide concentration is obtained to be 50% by weight of the oxidation solution, and the residual water due to washing is also to a certain extent when concentratio...
Embodiment 2
[0041]Under the condition of 100 DEG C and 0.3 MPa, and the volume content of tail oxygen is controlled to be less than 5%, cumene and air are oxidized to obtain cumene hydroperoxide (CHP) oxidation solution with a weight concentration of 20-24%.
[0042] Using Na 2 CO 3 Wash the cumene hydroperoxide oxidation solution with an aqueous solution with a weight content of 5%, wherein the volume ratio of the oxidation solution to the alkali solution is 4:1, and remove the organic acid therein. Then wash the oxidizing solution with deionized water to remove the residual Na due to alkaline washing + , wherein the volume ratio of oxidizing solution to deionized water is 4:1. According to the needs of the epoxidation reaction, it is concentrated in vacuum, the concentration temperature is 70°C, and the cumene hydroperoxide concentration is obtained to be 40% by weight of the oxidation solution, and the residual water due to washing is also to a certain extent when the concentration i...
Embodiment 3
[0047] Under the conditions of 98° C. and 0.3 MPa and controlling the volume content of tail oxygen to be lower than 5%, the cumene and air are oxidized to obtain a cumene hydroperoxide (CHP) oxidation solution with a weight concentration of 20-24%.
[0048] Wash the cumene hydroperoxide oxidation solution with an aqueous solution with a weight content of 2% NaOH, wherein the volume ratio of the oxidation solution to the alkali solution is 3:1, and remove the organic acid therein. Then wash the oxidizing solution with deionized water to remove the residual Na due to alkaline washing + , wherein the volume ratio of oxidizing solution to deionized water is 4:1. According to the needs of the epoxidation reaction, vacuum enrichment is carried out to it, and the enrichment temperature is 80° C., and the cumene hydroperoxide concentration is obtained to be 60% by weight of the oxidized liquid. When enriching, the residual water due to washing is also to a certain extent above is re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com