An electrolytic cell for electrochemical reduction of carbon dioxide and its application
A carbon dioxide and electrolytic cell technology, applied in the direction of electrolytic process, electrolytic components, electrolytic organic production, etc., can solve the problems of ineffective improvement of CO2 conversion rate, unsuitable for large-scale application, intensified side reaction of hydrogen evolution, etc., to expand the electrochemical reaction area, increase the electrochemical reaction speed, and control the effect of simplicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
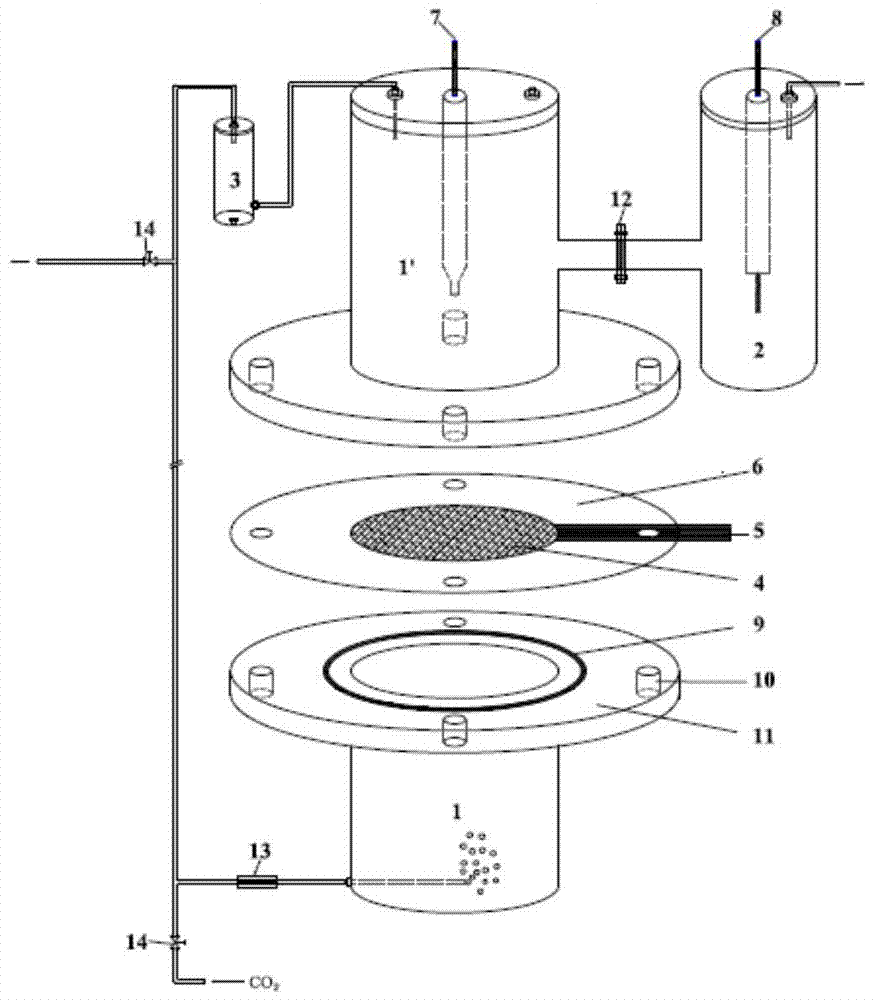
[0062] 1) Seal the foamed copper material with a porosity of 85%, a pore diameter of 0.1 mm, and a thickness of 1 mm with a material coated with a thermosetting resin to obtain a working electrode with an exposed geometric area of 3 cm 2 .
[0063] 2) Place the working electrode between the flanges of the upper and lower chambers of the cathode chamber made of plexiglass with a thickness of 4mm, use silica gel material as the sealing element, and place the upper chamber, the working electrode and the lower chamber of the cathode chamber Make connections and fastenings to ensure that there is no leakage of liquids and gases;
[0064] 3) The cathode chamber is connected with the anode chamber made of plexiglass, and the NF115 produced by Dupont is used as the ion exchange membrane, and the seal is checked until there is no gas and liquid leakage;
[0065] 4) Pour 0.5M NaHCO into the cathode chamber 3 Aqueous solution, pour 0.1M H into the anode chamber 2 SO 4 aqueous solut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com