Pyrrolo quinoline quinone (PQQ) disodium salt impurity separation and purification method
A technology for separation and purification, quinoline quinone, applied in the field of chemical drug impurity research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Embodiment 1. The investigation of sample solubility
[0053] Since the preparative liquid chromatography requires a large injection volume, the sample concentration is too low to cause the injection volume to be too large, and the high-concentration solute is overloaded, and the peak expansion caused by the large volume can be avoided during the separation process. Therefore, it is necessary to find a suitable solvent to achieve a certain solubility in order to effectively separate on the preparative chromatographic column and obtain a certain amount of preparation.
[0054]Use deionized water, methanol, acetonitrile (CAN), 0.04M ammonium dihydrogen phosphate buffer solution (pH7.0) (Buffer) containing 10% tetrabutylammonium hydroxide (Buffer), Buffer / ACN (7:3), and investigate PQQ in Solubility in different solvents. The results are shown in the table below:
[0055]
[0056] When the 0.04M ammonium dihydrogen phosphate buffer solution (pH7.0) containing 10% tetr...
Embodiment 2
[0057] Embodiment 2. optimize preparation and separation conditions
[0058] The preparative chromatographic conditions in chemical synthesis are generally selected from the reference analytical column chromatographic conditions, and then expanded to the same type of preparative column separation. For the extraction and purification of unknown samples, analytical columns are usually used to explore the separation conditions, and then the separation is scaled up. In general, the separation degree of preparative chromatography should be greater than 1.25. Select the preparative chromatographic column, enlarge the injection volume, flow rate, etc. according to the scale of the preparation, and finally make appropriate adjustments in the preparative chromatography.
[0059] Preparative chromatography operates in the nonlinear range and belongs to nonlinear chromatography, while analytical chromatography is linear chromatography. Therefore, many concepts or parameters used in anal...
Embodiment 3
[0071] Example 3 The separation on the preparative column
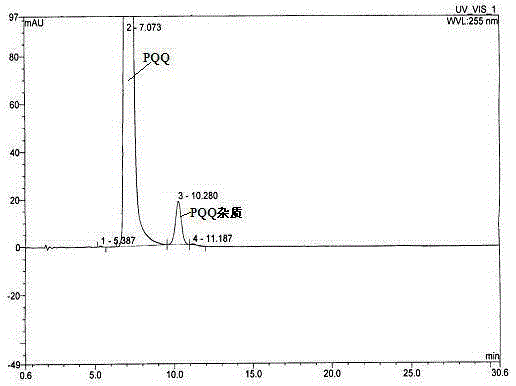
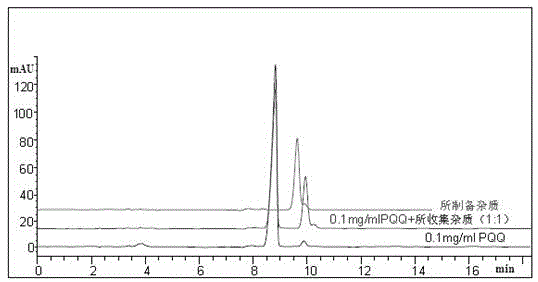
[0072] Because this test is to obtain impurities with extremely low content, high value and difficult to separate. Therefore, columns with small particles (5 μm) and large inner diameters are used to optimize the separation by adjusting the relevant parameters in the separation equation. Due to the particularity of PQQ molecules, the separation curve changes during the gradual amplification process. We finally choose the column: GPC18 (5μm, 21.2×250mm), and slightly adjust the mobile phase: 0.04M containing 10% tetrabutylammonium hydroxide Ammonium dihydrogen phosphate buffer solution (pH7.0): ACN=75:25 (v / v); column temperature: room temperature; flow rate: 20mL / min; injection volume: 10mL; pressure: 1756psi; detection wavelength: 225nm.
[0073] 3.1 Selection of flow rate
[0074] The production yield increases with the increase of column length and mobile phase flow rate. Since we are using a column with small p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com