Dexzopiclone oral fast-dissolving film and preparation method thereof
A technology of eszopiclone and oral instant film, which is applied to pharmaceutical formulations, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Simple process, pure sweetness effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
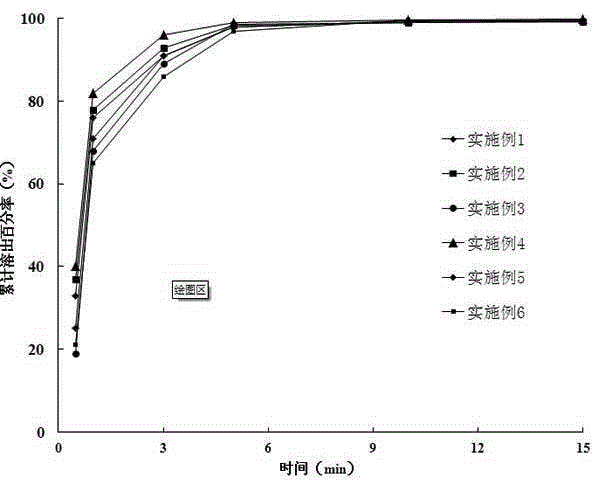
Image
Examples
Embodiment 1
[0038] Prescription composition:
[0039]
[0040]
[0041] The eszopiclone inclusion complex is prepared by spray drying with eszopiclone and β-cyclodextrin in a molar ratio of 1:1.
[0042] Preparation Process:
[0043] (1) Accurately weigh 29.2 g of β-cyclodextrin and 10.0 g of eszopiclone with a molar ratio of 1:1, respectively, heat the β-cyclodextrin with water to 50°C and dissolve it into a saturated solution. The clone was dissolved in 150 ml of acetone and slowly added to the above saturated solution of β-cyclodextrin, stirred in a constant temperature water bath at 65°C for 4-6 hours, and then the solution was spray-dried in a spray dryer to obtain eszopiclone inclusion complex powder;
[0044] (2), accurately weigh the recipe quantity KollicoatProtect, dissolve in purified water under heating and stirring conditions at 60~70 ℃, then add malic acid, sucralose and menthol in turn, and stir to obtain blank glue;
[0045] (3), the eszopiclone clathrate powder in...
Embodiment 2
[0048] Prescription composition:
[0049]
[0050] The eszopiclone inclusion complex is prepared by spray drying with eszopiclone and β-cyclodextrin in a molar ratio of 1:1.
[0051] Preparation Process:
[0052] (1) Accurately weigh 29.2 g of β-cyclodextrin and 10.0 g of eszopiclone with a molar ratio of 1:1, respectively, heat the β-cyclodextrin with water to 70°C and dissolve it into a saturated solution. The clone was dissolved in 150 ml of acetone and slowly added to the above saturated solution of β-cyclodextrin, stirred in a constant temperature water bath at 65°C for 4-6 hours, and then the solution was spray-dried in a spray dryer to obtain eszopiclone inclusion complex powder;
[0053] (2), accurately weigh the recipe quantity PVAMowiol4-88, dissolve in purified water under heating and stirring conditions at 60~70°C, then add sorbitol, PEG400, citric acid, sucralose and lemon essence successively, stir well to obtain blank glue;
[0054] (3), take the eszopiclo...
Embodiment 3
[0057] Prescription composition:
[0058]
[0059] The eszopiclone inclusion complex is prepared by spray drying with eszopiclone and β-cyclodextrin in a molar ratio of 1:1.
[0060] Preparation Process:
[0061] (1), respectively accurately weigh 29.2 g of β-cyclodextrin and 10.0 g of eszopiclone with a molar ratio of 1:1, heat the β-cyclodextrin with water to 60°C to dissolve it into a saturated solution, and eszopiclone The clone was dissolved in 150 ml of acetone and slowly added to the above saturated solution of β-cyclodextrin, stirred in a constant temperature water bath at 65°C for 4-6 hours, and then the solution was spray-dried in a spray dryer to obtain eszopiclone inclusion complex powder;
[0062] (2), accurately weigh the recipe quantity HPMCE5, dissolve in purified water under heating and stirring conditions at 60~70 ℃, then add glycerin, tartaric acid, sucralose and sweet orange essence successively, and stir to obtain blank glue;
[0063] (3), disperse th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com