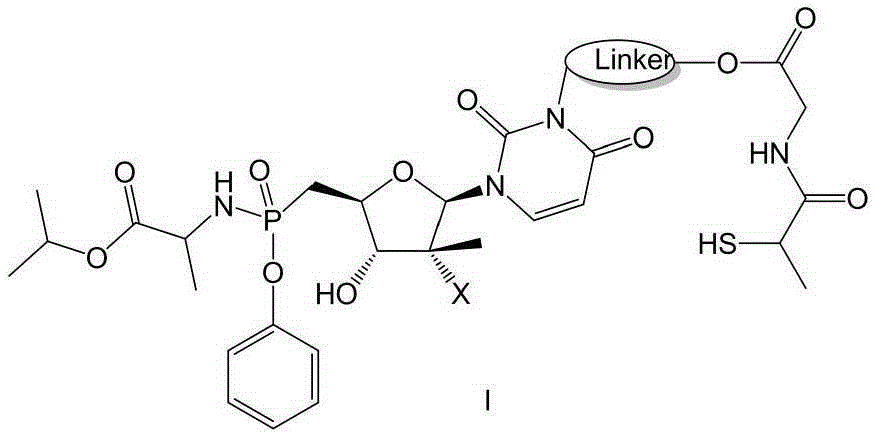
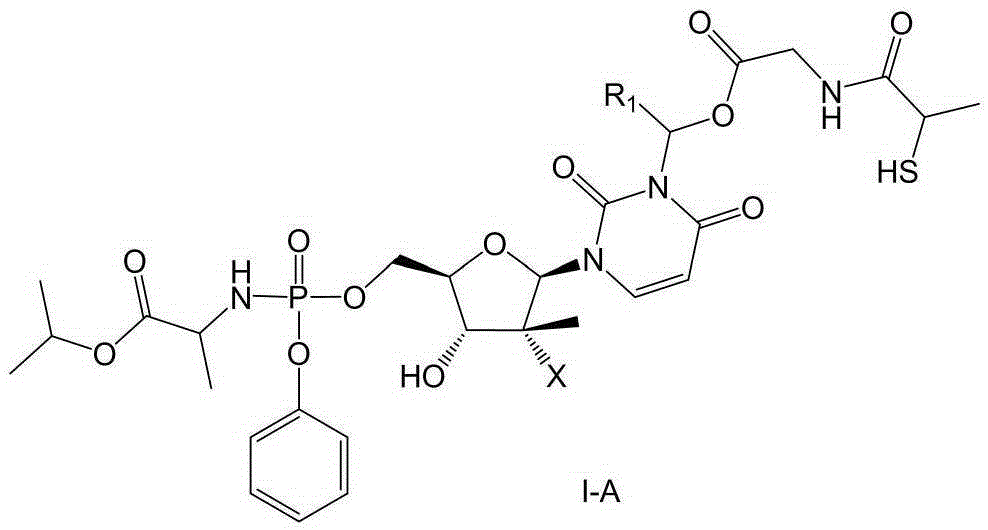
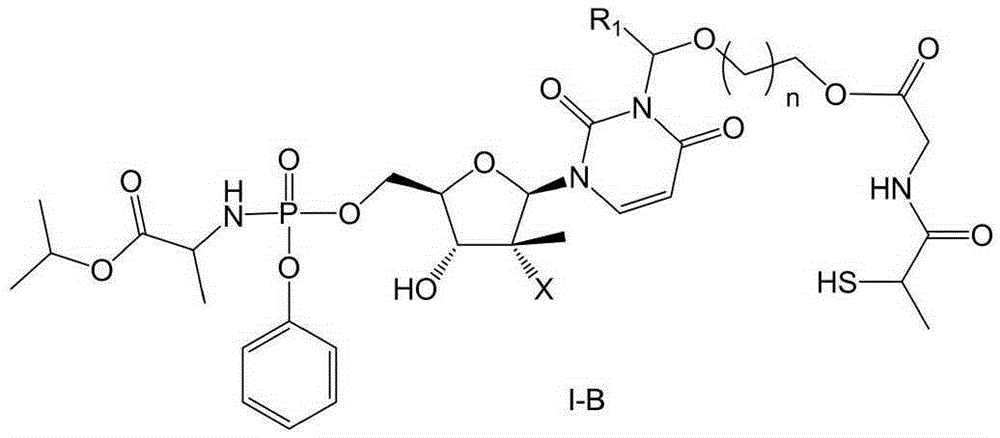
Prodrug containing tiopronin structure, preparation method of prodrug, pharmaceutical composition and application of pharmaceutical composition
A tiopronin and prodrug technology, applied in the field of medicinal chemistry, can solve the problems of poor patient compliance and increased financial burden on patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Embodiment 1: the synthesis of compound II-A-1
[0077]
[0078] step one:
[0079] Dissolve 529mg (1.0mmol) of III-1 in 10mL of anhydrous acetone, add 680mg (1.5mmol) of IV-1 and 414mg (3.0mmol) of potassium carbonate at room temperature, heat, stir and reflux for 6h, and TLC detects that the reaction is complete. After filtration, the filtrate was diluted with dichloromethane (30 mL), washed with water (10 mL) and saturated brine (10 mL) successively, the organic phase was separated, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography to obtain 710 mg of white solid V-1 with a yield of 75%. 1 HNMR (400MHz, CDCl 3 )δ8.90(s,1H),7.51–7.38(m,7H),7.35–7.25(m,9H),7.22(dd,J=10.2,4.2Hz,5H),7.17(d,J=7.6Hz ,1H),6.41(dt,J=72.5,5.3Hz,1H),6.15(d,J=17.9Hz,1H),5.52(d,J=8.2Hz,1H),5.17(dd,J=19.9, 8.7Hz, 1H), 4.97(dq, J=12.6, 6.3Hz, 1H), 4.49(dt, J=5.4, 4.7Hz, 1...
Embodiment 2
[0083] Embodiment 2: the synthesis of compound II-A-2
[0084]
[0085] step one:
[0086]Dissolve 529mg (1.0mmol) III-1 in 10mL of anhydrous acetone, add 680mg (1.5mmol) IV-2 and 414mg (3.0mmol) potassium carbonate at room temperature, heat, stir and reflux for 6h, and TLC detects that the reaction is complete. After filtration, the filtrate was diluted with dichloromethane (30 mL), washed with water (10 mL) and saturated brine (10 mL) successively, the organic phase was separated, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography to obtain 306 mg of white solid V-1 with a yield of 38%.
[0087] 1 HNMR (400MHz, CDCl 3 )δ8.90(s,1H),7.51-7.38(m,7H),7.35-7.25(m,9H),7.22(dd,J=10.2,4.2Hz,5H),7.17(d,J=7.6Hz ,1H),6.65(q,J=6.5Hz,1H),6.19(d,J=17.9Hz,1H),5.10-4.94(m,1H),4.52(dd,J=11.4,5.7Hz,1H) ,4.39-4.21(m,3H),4.22-4.05(m,2H),3.98(tt,J=11.5,5.8Hz,1H),3.57-3....
Embodiment 3
[0091] Embodiment 3: the synthesis of compound II-B-1
[0092]
[0093] step one:
[0094] Dissolve 529mg (1.0mmol) of III-1 in 10mL of anhydrous acetone, add 690mg of IV-3 and 414mg (3.0mmol) of potassium carbonate at room temperature, heat, stir and reflux for 6h, and TLC detects that the reaction is complete. After filtration, the filtrate was diluted with dichloromethane (30 mL), washed with water (10 mL) and saturated brine (10 mL) successively, the organic phase was separated, dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to remove the solvent. The residue was purified by silica gel column chromatography to obtain 300 mg of V-3, which was directly used in the next reaction.
[0095] Step two:
[0096] Dissolve 200 mg (0.21 mmol) of V-3 in 10 mL of anhydrous dichloromethane, add 2 mL (volume ratio: dichloromethane / triisopropylsilane / trifluoroacetic acid=5 / 1 / 1) solution at room temperature, and react After about 1 h, TLC detec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com