A kind of method for preparing rh-based catalyst and rh-based catalyst and application
A catalyst and promoter technology, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, etc. The effect of handling, safe and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Preparation of catalyst
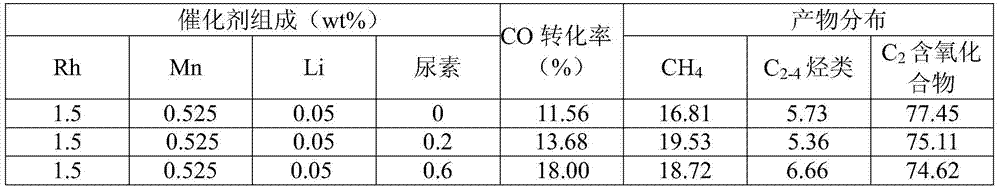
[0029] 0.154g RhCl 3 ·3H 2 O, 0.0756g MnCl 2 4H 2 O, 0.01726g LiCl·H 2 O and 0.024g urea are dissolved with 15ml deionized water, and the percentage composition corresponding to the carrier of each component is respectively 1.5wt%, 0.525wt%, 0.05wt% and 0.6wt%; Weigh 4g silica gel (20~40 orders) and added to the above impregnating solution, stirred at room temperature for 1 hour and left to dry; put the dried catalyst precursor in an oven at 100°C for 12 hours to further remove the moisture in the catalyst precursor; dry the The catalyst precursor was taken out and placed in a muffle furnace from room temperature to the roasting temperature at a heating rate of 3°C / min, and then roasted at a roasting temperature of 300°C for 3 hours; the roasted catalyst precursor was taken out and placed in In a tube furnace, the reduction is carried out under hydrogen, and the temperature is raised from room temperature to the reduction temperature a...
Embodiment 2
[0039] (1) Preparation of catalyst
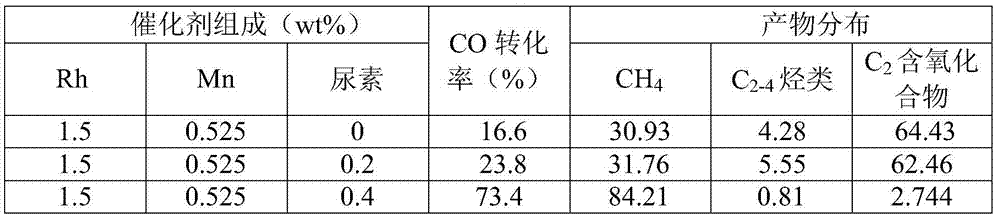
[0040] 0.154g RhCl 3 ·3H 2 O, 0.0756g MnCl 2 4H 2 O and 0.016g urea were dissolved in 15ml of deionized water, and the percentages of each component corresponding to the carrier were 1.5wt%, 0.525wt% and 0.4wt% respectively; 4g of silica gel (20-40 mesh) was weighed and added to the above In the impregnating solution, stir at room temperature for 1 hour and let it dry in the air; put the dried catalyst precursor in an oven at 100°C for 12 hours to further remove the moisture in the catalyst precursor; put the dried catalyst precursor Take it out and put it in a muffle furnace for roasting at 300°C, the roasting time is 3 hours, and the heating rate is 3°C / min; take out the roasted catalyst precursor and put it in a tube furnace, and perform reduction under hydrogen at a reduction temperature of 350 ℃, the heating rate is 2℃ / min, the reduction time is 2h, and the hydrogen space velocity is 600ml·g -1 h -1 ; When the temperature in the ...
Embodiment 3
[0050] (1) Preparation of catalyst
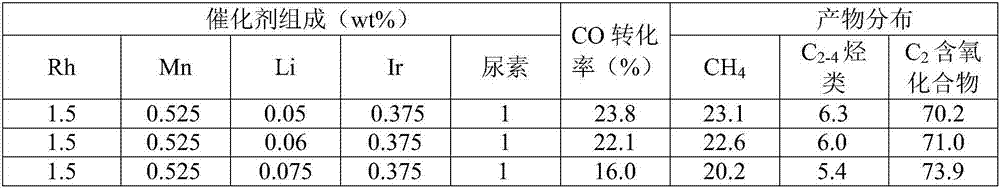
[0051] 0.154g RhCl 3 ·3H 2 O, 0.0756g MnCl 2 4H 2 O, 0.01726g LiCl·H 2 O, 0.0233gIrCl 3 and 0.04g urea are dissolved with about 15ml of deionized water, and the percentages of each component corresponding to the carrier are respectively 1.5wt%, 0.525wt%, 0.05wt%, 0.375wt% and 1.0wt%; weigh 4g silica gel ( 20 to 40 mesh) and added to the above impregnating solution, stirred at room temperature for 1 hour and left to dry; put the dried catalyst precursor in an oven at 100°C for 12 hours to further remove the catalyst precursor Moisture; take out the dried catalyst precursor and put it in a muffle furnace for roasting at 300°C, the roasting time is 3h, and the heating rate is 3°C / min; take out the roasted catalyst precursor and put it in a tube furnace, The reduction is carried out under hydrogen, the reduction temperature is 350°C, the heating rate is 2°C / min, the reduction time is 2h, and the hydrogen space velocity is 600ml·g -1 h -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com