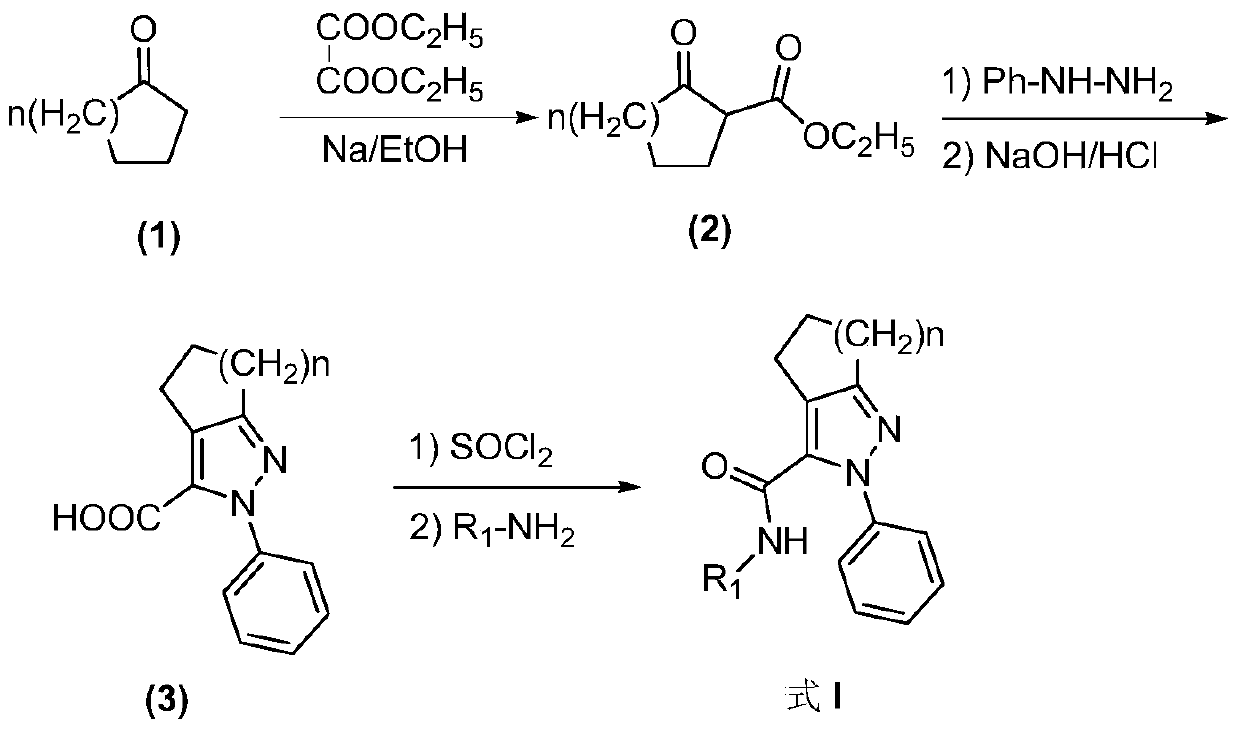
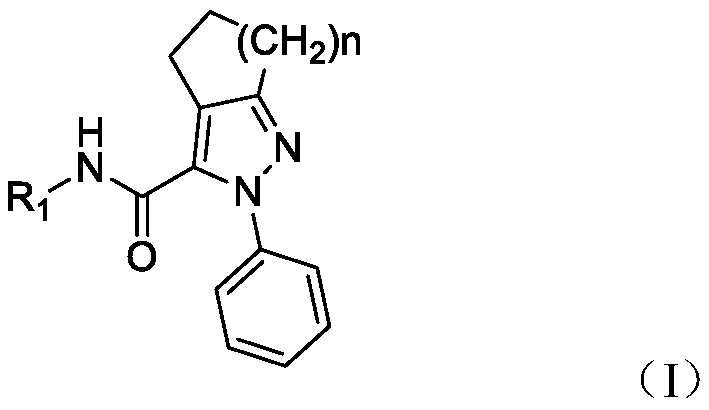
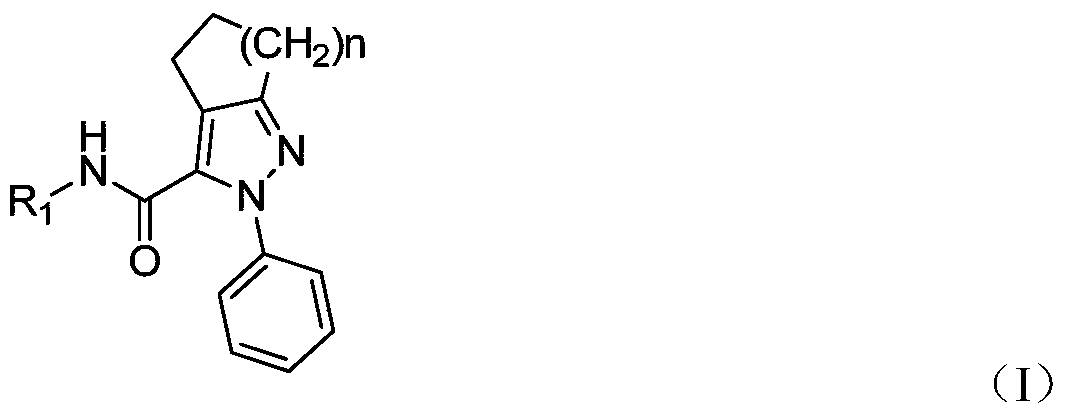Pyrazolo-3-carboxamide analogs and their preparation and application
A formamide, pyrazolo technology, applied in the field of organic compounds, can solve the problems of poor stability, difficult synthesis, complex chemical structure and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: N-(3-methoxyphenyl)-2-phenyl-2,4,5,6-tetrahydrocyclopenta[c]pyrazole-3-carboxamide (I2)
[0035] Step 1: Preparation of ethyl 2-oxocyclopentyl acetylacetonate
[0036] Add 2.05g of sodium to 100ml of absolute ethanol, after the sodium is dissolved, heat and keep at 40°C for 1h. Cool to 0°C, slowly add dropwise a mixture of 17.5g (0.12mol) diethyl oxalate and 10.0g (0.12mol) cyclopentanone, and stir for 4h at a temperature lower than 4°C. Add 100ml of ice water, adjust the pH to 2 with 36.5% hydrochloric acid, extract with dichloromethane, dry and filter the organic phase, and remove the solvent to obtain 10.0g of yellow liquid with a yield of 45%.
[0037] Step 2: Preparation of 2-phenyl-2,4,5,6-tetrahydrocyclopenta[c]pyrazole-3-carboxylic acid
[0038] Add 0.69g (0.005mol) of phenylhydrazine hydrochloride and 10ml of ethanol to a 100ml three-necked flask, add an appropriate amount of triethylamine under stirring to adjust the pH to 7, and slowly add 1.0g (...
Embodiment 2
[0043] Example 2: N-(4-tert-butylphenyl)-2-phenyl-4,5,6,7-tetrahydrocyclohexane[c]pyrazole-3-carboxamide (I3)
[0044] Step 1: Preparation of Ethyl 2-Oxycyclohexylacetylacetonate
[0045] Add 2.05g of sodium to 100ml of absolute ethanol, after the sodium is dissolved, heat and keep at 40°C for 1h. Cool to 0°C, slowly dropwise add a mixture of 14.6g (0.10mol) diethyl oxalate and 10.0g (0.10mol) cyclohexanone, and stir for 4h at a temperature lower than 4°C. Add 100ml of ice water, adjust the pH to 2 with 36.5% hydrochloric acid, extract with dichloromethane, dry and filter the organic phase, and remove the solvent to obtain 9.6g of yellow liquid with a yield of 48%.
[0046] Step 2: Preparation of 2-phenyl-4,5,6,7-tetrahydrocyclohexane[c]pyrazole-3-carboxylic acid
[0047] Add 0.69g (0.005mol) of phenylhydrazine hydrochloride and 10ml of ethanol to a 100ml three-necked flask, add an appropriate amount of triethylamine under stirring to adjust the pH to 7, and slowly add 1.0g ...
Embodiment 3
[0052] Example 3: N-(4-tert-butylphenyl)-2-phenyl-2,4,5,6,7,8-hexahydrocyclohepta[c]pyrazole-3-carboxamide (I5)
[0053] Step 1: Preparation of Ethyl 2-Oxycycloheptylacetylacetonate
[0054] Add 2.05g of sodium to 100ml of absolute ethanol, after the sodium is dissolved, heat and keep at 40°C for 1h. Cool to 0°C, slowly add dropwise a mixture of 13.0g (0.09mol) diethyl oxalate and 10.0g (0.09mol) cycloheptanone, and stir for 4h at a temperature lower than 4°C. Add 100ml of ice water, adjust the pH to 2 with 36.5% hydrochloric acid, extract with dichloromethane, dry and filter the organic phase, and precipitate from the solvent to obtain 12.7g of dark red viscous liquid, with a yield of 67.1%.
[0055] Step 2: Preparation of 2-phenyl-2,4,5,6,7,8-hexahydrocyclohepta[c]pyrazole-3-carboxylic acid
[0056] Add 0.69g (0.005mol) of phenylhydrazine hydrochloride and 10ml of ethanol to a 100ml three-necked flask, add an appropriate amount of triethylamine to adjust the pH to 7 while ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com