Two staphylococcus aureus protein antigens and preparation method and use thereof
A Staphylococcus, aureus technology, applied in the fields of molecular biology and immunology, can solve the problems of small expression and difficult purification steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1: Synthesis of Nucleotide Sequence Encoding Staphylococcus aureus alpha-hemolysin Attenuated Protein and Obtaining of Clones Containing the Same
[0054] The amino acid sequence of natural Staphylococcus aureus α-hemolysin (GenBank accession number: AAA26598.1) was obtained through network search, as shown in SEQ ID NO: 1. The sequence was modified as follows: histidine (His, H) at position 35 was replaced by leucine (Leu, L) to obtain the S. aureus alpha-hemolysin mutation shown in SEQ ID NO: 3 The amino acid sequence of the body (HlaM35).
[0055] The nucleotide sequence encoding the amino acid sequence of HlaM35 was optimized by selecting the preferred codons of Escherichia coli, and the nucleotide sequence of SEQ ID NO: 4 was obtained by chemical synthesis. NdeI was added upstream of the nucleotide sequence of SEQ ID NO: 4, and an XhoI restriction site was added downstream. Thereby a nucleotide sequence for protein expression is obtained.
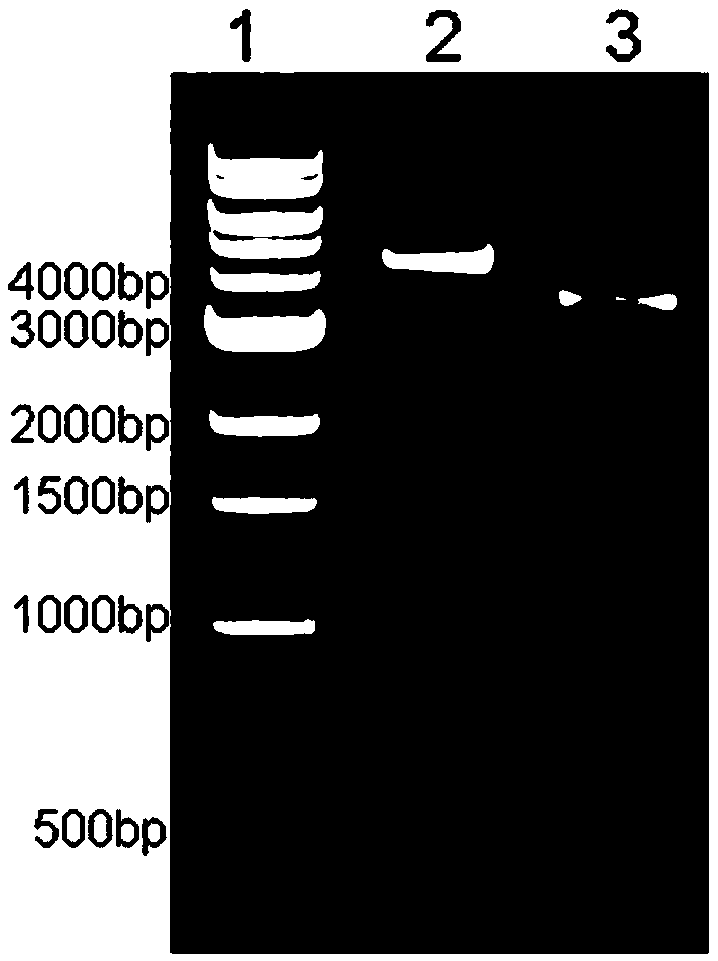
[0056] The ob...
Embodiment 2
[0057] Example 2. Obtainment of the nucleotide sequence encoding native S. aureus alpha-hemolysin (HlaWT) and obtaining of clones containing the same
[0058] The primers were designed, and the nucleotide sequence shown in SEQ ID NO: 4 was used as a template for mutation by the method of PCR to obtain the nucleotide sequence encoding HlaWT shown in SEQ ID NO: 2.
[0059] Mutation primers are as follows:
[0060] HlaWT-1:
[0061]5’-CGATAAAGAAAACGGTATGCACAAGAAAGTTTTTCTACTCTTTCA-3’
[0062] HlaWT-2:
[0063] 5’-TGAAAGAGTAGAAAACTTTCTTGTGCATACCGTTTTCTTTATCG-3’
[0064] PCR system: 1 μl (10 ng) of the PET20b-HlaM35 plasmid template DNA obtained above, 2 μl each of mutant primers HlaWT-1 and HlaWT-2 (0.5 μM concentration), 1 μl of dNTP, 0.5 μl of PfuTurbo DNA polymerase (2.5 U / μl, purchased from Takera), 5 μl of 10× reaction buffer, 38.5 μl of deionized water, and a total volume of 50 μl.
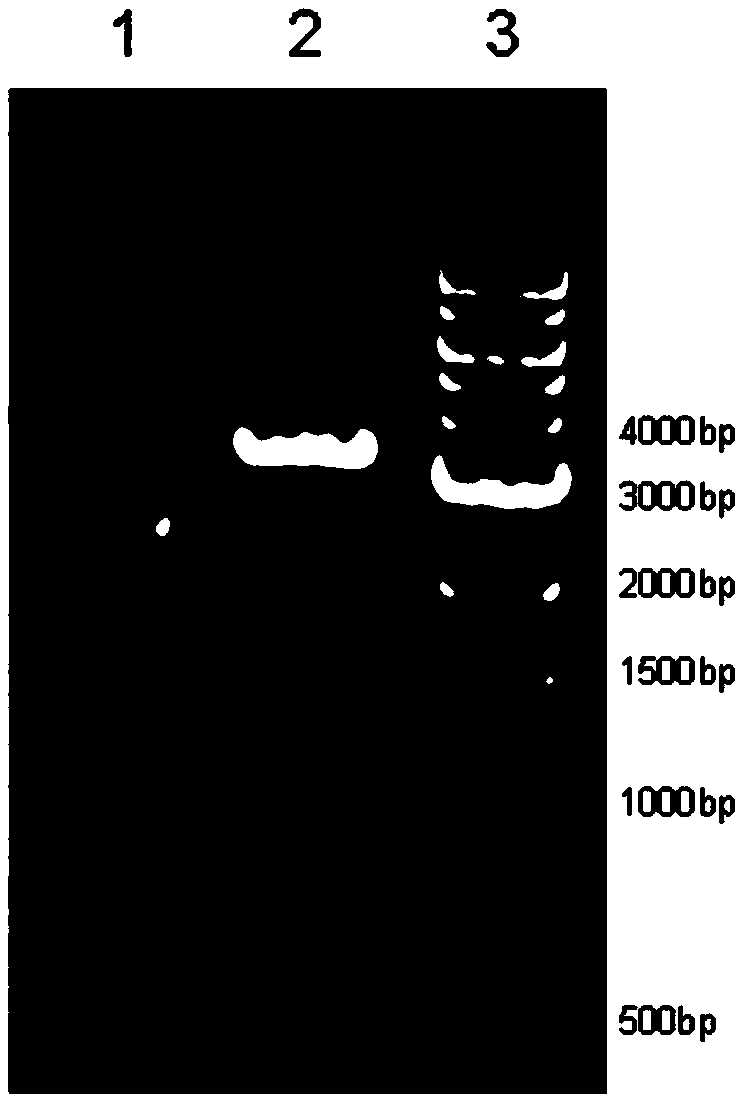
[0065] Mutation PCR conditions: preheating at 95°C for 5 min; 95°C, 1 min, 58°C, 1 min, ...
Embodiment 3
[0067] Example 3. Synthesis of Nucleotide Sequences Encoding Full Length IsdB Protein and Obtaining of Clones Containing the Same
[0068] The amino acid sequence of Staphylococcus aureus iron-regulated surface determinant protein B (IsdB) was obtained through network retrieval, as shown in SEQ ID NO: 5.
[0069] The preferred codons of Escherichia coli were selected to optimize the nucleotide sequence encoding the amino acid sequence of IsdB, and the nucleotide sequence encoding IsdB of SEQ ID NO: 6 was obtained by chemical synthesis. NdeI was added upstream of the nucleotide sequence of SEQ ID NO: 6, and an XhoI restriction site was added downstream. Thereby a nucleotide sequence that can be used for protein expression is obtained.
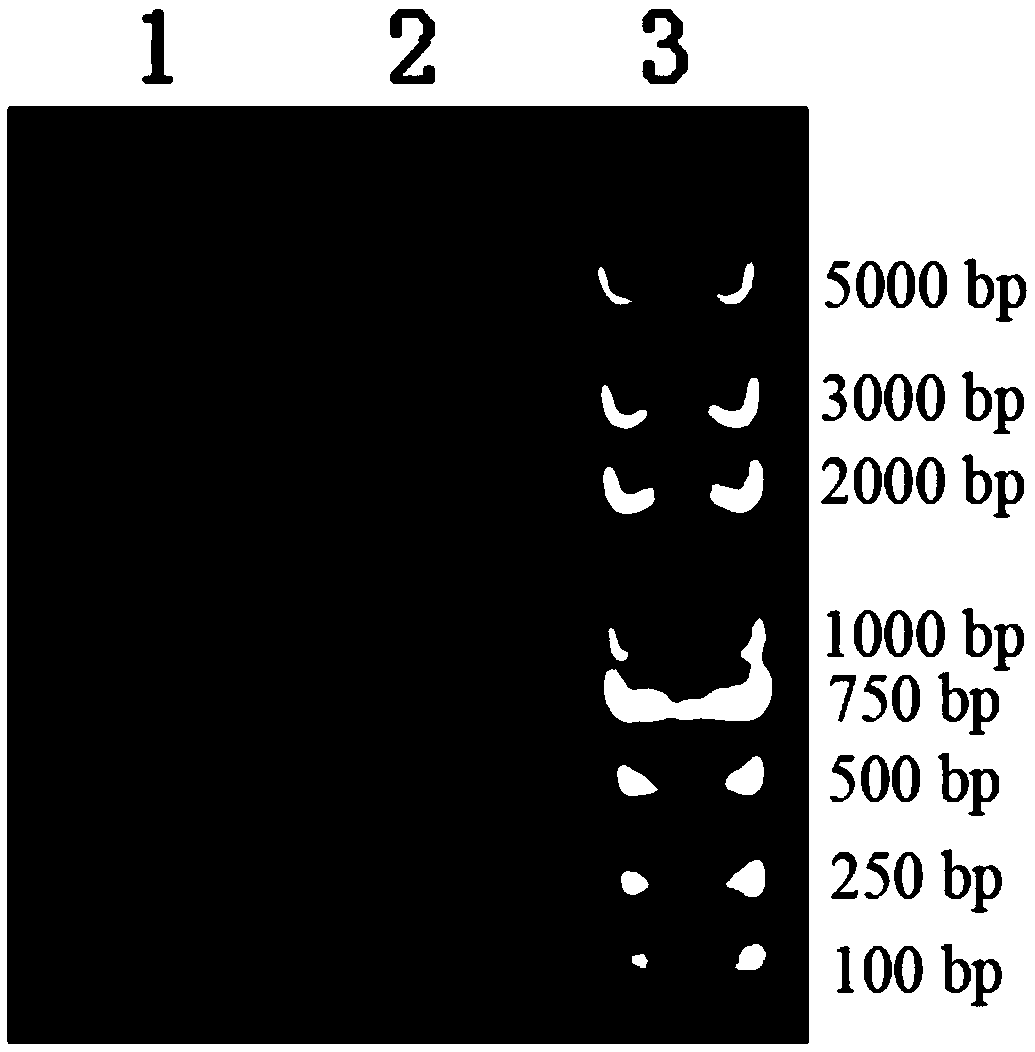
[0070] The obtained nucleotide sequence identified by sequencing was double digested with NdeI and XhoI, and then ligated with the expression vector PET20b (purchased from Novagen) that had been digested with the same double enzyme, and then li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com