Fusion protein related to HBV, preparing method thereof and application thereof
A technology of fusion protein and composition, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
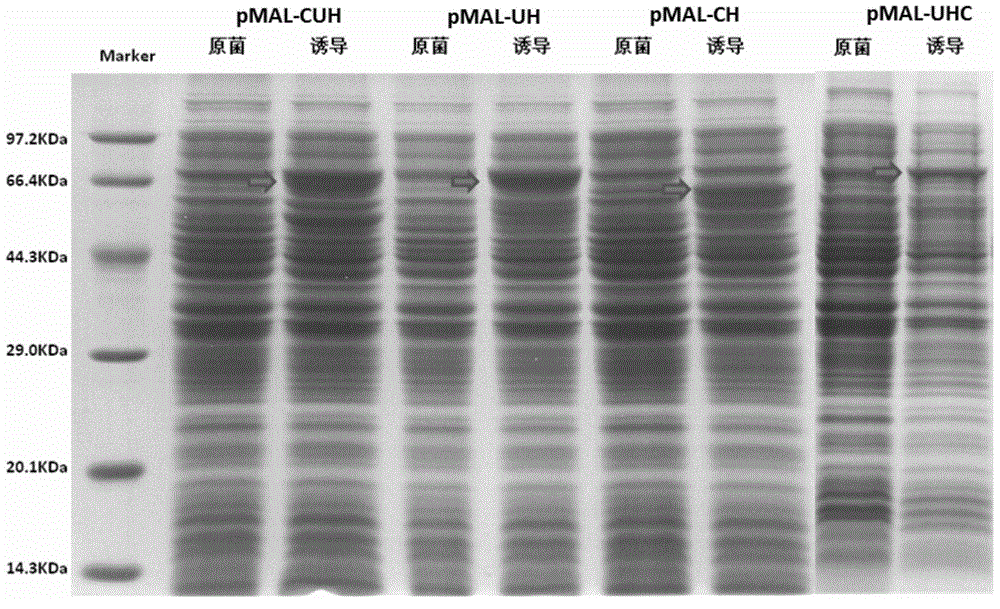
[0199] Construction and expression of embodiment 1CTP-Ub-HbcAg (CUH) and Ub-HbcAg-CTP (UHC) prokaryotic expression vector
[0200] 1. Gene synthesis
[0201] 1.1 PCR amplification was carried out after whole gene synthesis of CTP-Ub-HBcAg, Ub-HBcAg-CTP, Ub-HBcAg and CTP-HbcAg sequences.
[0202] According to the sequences of CTP-Ub-HBcAg, Ub-HBcAg-CTP, Ub-HBcAg and CTP-HBcAg, primers for whole gene synthesis were designed, and the synthesized fragments were named CUH, UHC, UH and CH, respectively. The primer synthesis company is Suzhou Jinweizhi Biotechnology Co., Ltd., and the primer sequences are as follows. Considering the construction of recombinant plasmids when designing primers, BamHI and XhoI restriction sites were introduced at both ends of the primers.
[0203] The attached primer sequences are shown in the table below:
[0204]
[0205]
[0206] 1.2 Gene synthesis
[0207] PCR amplification for gene synthesis
[0208] Synthesis of CTP-Ub-HBcAg (CUH): prim...
Embodiment 2
[0246] Example 2 In Vitro Activity Detection
[0247] method:
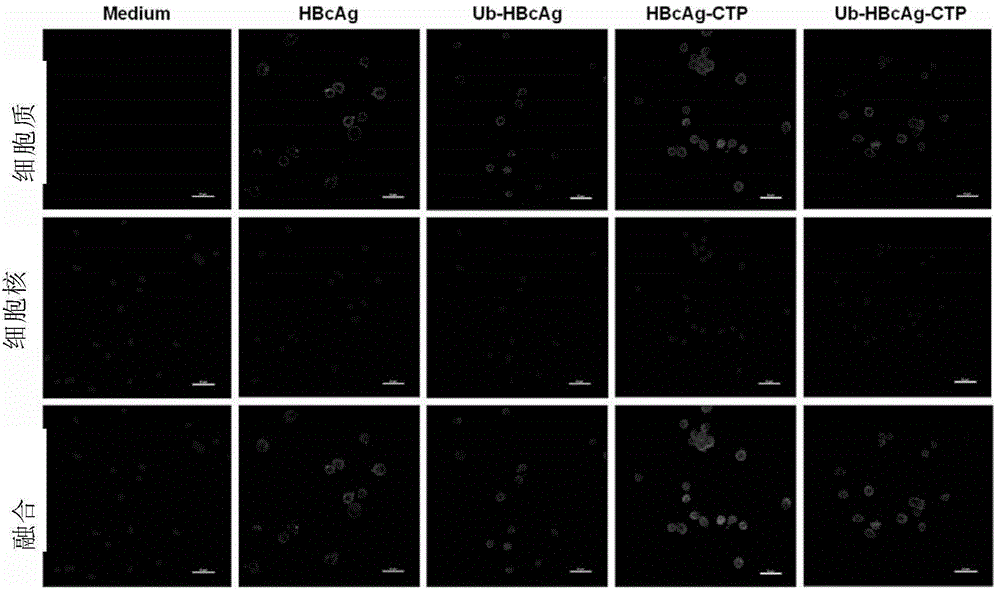
[0248] 1. Isolate and culture myeloid-derived DCs from inbred Balb / c mice in vitro, add rGM-CSF and rIL-4 and culture them for 5 days, then add LPS to induce DC maturation.
[0249] Different groups of fusion proteins were added to the cell culture medium, and the distribution and localization of immunofluorescence in the cells were observed under a laser confocal microscope, and the fluorescence intensity was quantitatively analyzed.
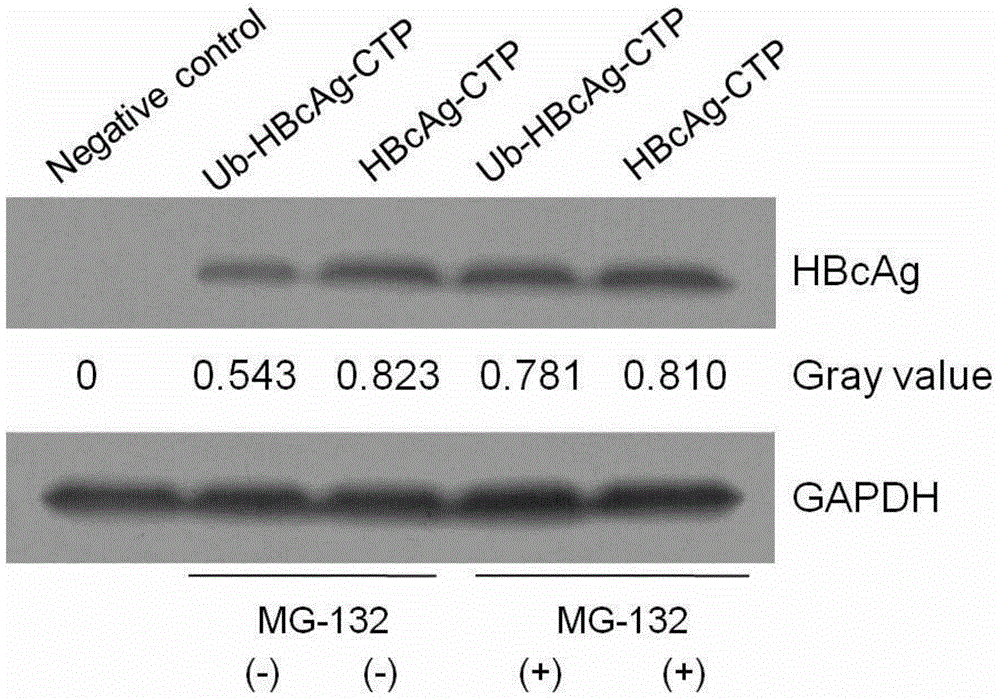
[0250] The transduction efficiency of Ub-HBcAg-CTP was detected by Western blot to observe the difference of HBcAg expression in different groups of cells. Antibodies used in Western blot were anti-HBcAg antibody (primary antibody) and goat anti-mouse secondary antibody (purchased from Wuhan Boster Company).
[0251] The expression of DC surface molecules was measured by flow cytometry, and fluorescently labeled with FITC-labeled mouse monoclonal antibodies CD11c, CD80, CD83, CD8...
Embodiment 3
[0263] Example 3 In vivo activity detection
[0264] method:
[0265] 1. In vivo activity experiment of BALB / c mice
[0266] BALB / c mice were randomly divided into experimental group Ub-HBcAg-CTP (20 μg), control group CTP-HBcAg (20 μg), HBcAg-Ub (20 μg), HBcAg (20 μg) and blank group (normal saline), after muscle immunization Mice, once a week, 3 times in total, 7 days after the last immunization, the following tests were performed:
[0267] Detection of Cytokines in T Lymphocytes by Flow Cytometry (FCM)
[0268] Enzyme-linked immunosorbent assay (ELISA) was used to detect cytokines secreted by T lymphocytes;
[0269] Cell counting kit (CCK-8) was used to detect the proliferative activity of T lymphocytes.
[0270] 2. In vivo activity experiment of HBV transgenic mice
[0271] HBV transgenic mice (purchased from the Liver Disease Center of the 458th Hospital of the Chinese People's Liberation Army, the HBV gene is integrated in the genome of the mouse, and the characteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com