Improved synthesis method of eslicarbazepine acetate
A technology of eslicarbazepine acetate and a synthesis method, applied in the field of medicinal chemistry, can solve the problems of high cost of reaction reagents, low conversion rate of raw materials, complicated post-processing, etc., and achieves reduction of production cost and environmental pollution, shortening of reaction steps, The effect of improving product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
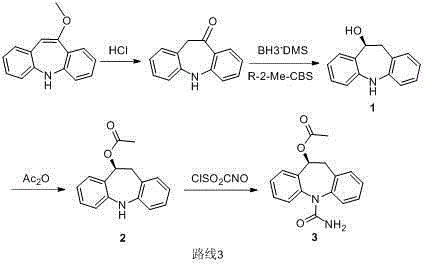
[0022] Add 200mL of toluene, 42g of intermediate 1 ((s)-10-hydroxyl-10,11dihydro-5H-dibenzo[b,f]azepine) and 33ml of triethylamine to a 500mL reaction flask, followed by Stir in an ice bath, then slowly add 17g of acetyl chloride dropwise, keep the temperature not exceeding 10°C, continue to keep warm for 30 minutes after the dropwise addition, TLC detection (developer: PE:EA=4:1) The reaction of raw materials is complete.
[0023] Add perfluorosulfonic acid resin to the system, continue to stir, and then dropwise add an aqueous solution of potassium cyanate (containing 18 g of potassium cyanate) into the system, raise the temperature to 70-80°C and react for 4 hours. A large amount of light yellow solid appeared in the system, filtered, and the filter cake was washed with water.
[0024] The solid obtained above was dissolved in 100 mL of ethyl acetate, the resin was removed by filtration, and then 400 mL of xylene was added dropwise to the obtained solution, during which mor...
Embodiment 2
[0027] The steps are the same as above, except that the acid-binding agent in step (1) is selected from sodium bicarbonate and pyridine. Finally, white powdery eslicarbazepine acetate was obtained with a yield of 91%. The product purity reaches 99.7% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com