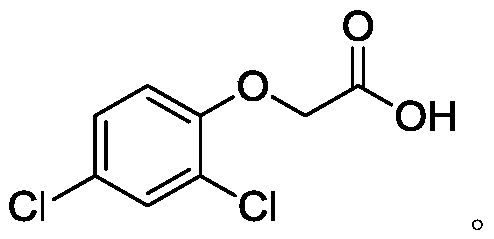
A New Synthetic Process of 2,4-Dichlorophenoxyacetic Acid
A technology of dichlorophenoxyacetic acid and methyl dichlorophenoxyacetate, applied in the field of chemical synthesis, can solve the problems of large amount of three wastes, high chlorination selectivity and high condensation yield, and achieves reduced production cost, good purity, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
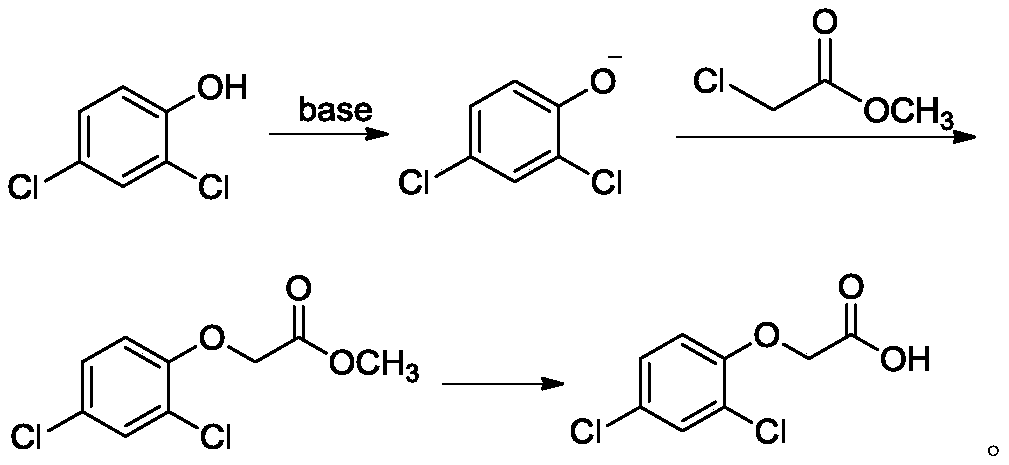
[0061] Mix and stir 83.2g (0.5mol) of 2,4-dichlorophenol, 416g toluene, and 116.7g (1mol) of KOH aqueous solution with a mass fraction of 48%, raise the temperature to 120°C, and react with water for 4 hours. After cooling to 110°C, 81.3 g (0.75 mol) of methyl chloroacetate was added dropwise, and the addition was completed in 4 hours, and the reaction was continued for 2 hours. Cool to 80°C, add 150g of water, adjust the pH of the reaction solution to 8, let it stand for stratification, add 120g of toluene to the water layer for extraction, add 10w.t.% sulfuric acid to the organic layer, wash it with rotary evaporation, and then add 300g of water for desolvation Dissolved to constant weight to obtain 105.2 g of the product with a purity of 99.5% and a yield of 89.1%.
Embodiment 3
[0063] 2,4-dichlorophenol 83.2g (0.5mol), chlorobenzene 100g, mass fraction 25% K 2 CO 3 276g (0.5mol) of aqueous solution, mixed and stirred, heated to 135°C, reacted with water for 3 hours, slightly cooled the neutralized solution to 100°C, added dropwise 43.4g (0.4mol) of methyl chloroacetate, and the dropwise addition was completed in 5 hours. The reaction was continued for 2 hours. Cool to 50°C, add 83.2g of water, adjust the pH of the reaction solution to 8, let it stand for stratification, add 16.6g of chlorobenzene to the water layer for extraction, add 30w.t.% acetic acid to the organic layer, wash it with rotary evaporation, and add 500g of water was precipitated to constant weight to obtain 108g of product with a purity of 99.3% and a yield of 91.3%.
[0064] Reference Example 4:
[0065] Mix and stir 83.2g (0.5mol) of 2,4-dichlorophenol, 416g xylene, and 75g (0.6mol) of NaOH aqueous solution with a mass fraction of 32%, raise the temperature to 145°C, and react ...
Embodiment 1
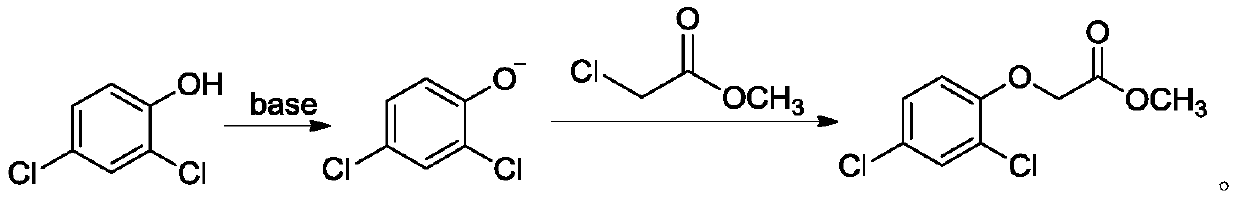
[0081] Embodiments 1 to 6 and comparative examples 1 to 3 are the preparation process of 2,4-dichlorophenoxyacetic acid, and its raw material 2,4-dichlorophenoxyacetic acid methyl ester is prepared by the method of reference example 1, and the reaction The formula is as follows:
[0082]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com