Method for preparing 1,1,1,3,3-pentachlorobutane
A technology of pentachlorobutane and chloropropene, applied in the field of preparation of 1,1,1,3,3-pentachlorobutane, which can solve the problems of high reaction temperature, difficult storage, and easy oxidation of cuprous chloride by air , to achieve the effect of mild reaction conditions and easier storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The main catalyst ferric chloride 8.13g (0.05mol), the cocatalyst tributyl phosphate 39.9g (0.15mol), the organic activator 1,1-azobiscyclohexanenitrile 12.2 (0.05mol) and carbon tetrachloride 770g (5mol) were sequentially added to a 1L stainless steel autoclave. Seal the autoclave, start stirring, and replace the air in the autoclave with nitrogen for three times. Subsequently, 156.25 g of 2-chloropropene was added at one time, the temperature of the reactor was heated to 80° C., and the reaction was completed after 1 hour. After the autoclave was lowered to room temperature, the autoclave was unloaded and the material was taken out. A liquid sample was taken with a pipette and analyzed by chromatography. The conversion rate of 2-chloropropene was 96%, and the selectivity of HCC-360jfa was 97%.
Embodiment 2
[0022] The operating process of embodiment 2 is similar to embodiment 1, and difference is that main catalyst is ferric bromide. The product was analyzed by chromatographic method, the conversion rate of 2-chloropropene was 94.5%, and the selectivity of HCC-360jfa was 94.3%.
Embodiment 3~9
[0024] The operating process of Examples 3-9 is similar to that of Example 1, except that the cocatalyst and organic activator are changed, and the reaction temperature is adjusted to match the activity of the telomerization catalyst. The reaction results are shown in Table 1.
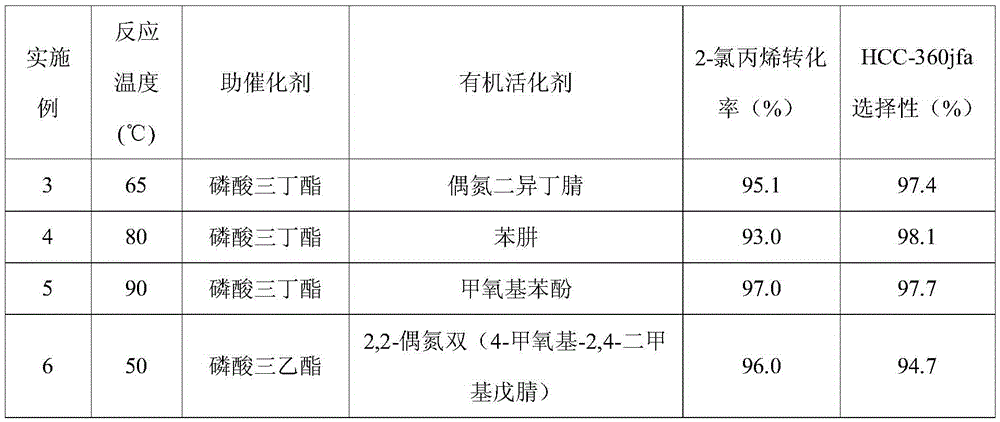
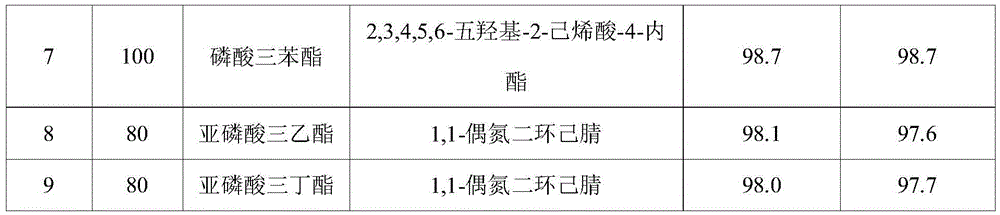
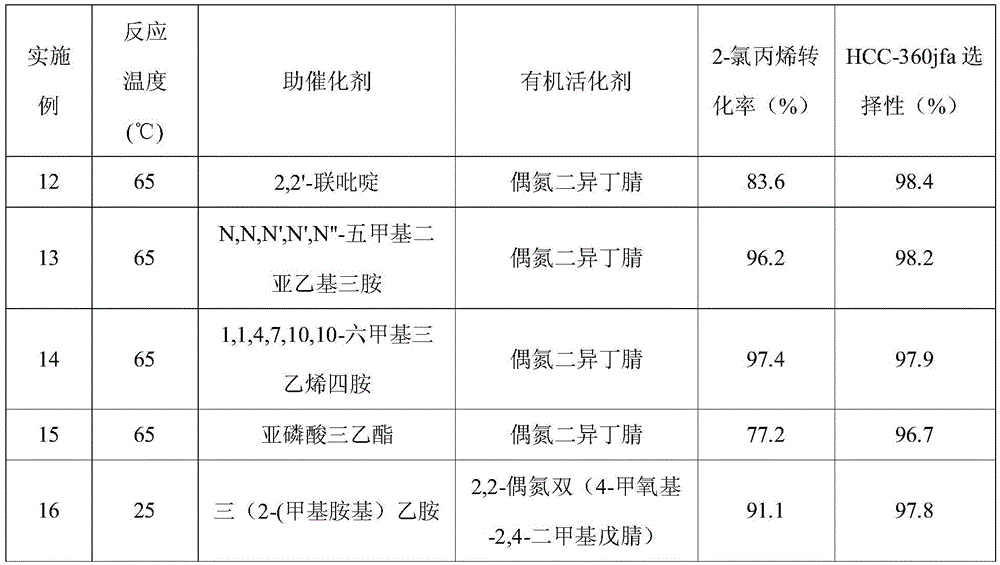
[0025] Table 1
[0026]
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com