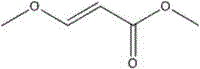
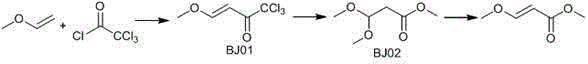Synthesizing method for 3-methoxyacrylate
A technology for the synthesis of methyl methoxyacrylate, applied in the field of chemistry, can solve the problems of increased separation cost and process, high comprehensive cost of industrialization, and many steps, and achieve good product purity, reduced production cost, and fewer reaction steps Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] (1) In a 500ml three-neck flask, add 182g of trichloroacetyl chloride and 300mL of dichloromethane, and cool down to -5°C to 0°C in an ice-salt bath. Slowly feed 120g of vinyl methyl ether, after ventilation is complete, stir and react at room temperature for 12 hours, take a sample and control, trichloroacetyl chloride is basically reacted completely, dichloroethane and excess vinyl methyl ether are distilled out under reduced pressure by the water pump, and then, the oil pump Distilled under reduced pressure to obtain 190g of BJ01, based on trichloroacetyl chloride, the yield was 85%.
[0029] (2) Add 190g of BJ01 and 250g of anhydrous methanol to a 1000ml three-neck flask, cool down to 0°C-10°C in an ice-salt bath, add 20g of anhydrous potassium carbonate, stir, heat to 20°C-30°C, and keep warm for 12 Hour. After the reaction is complete, cool down to room temperature, filter with suction to remove solid salts, distill the filtrate under reduced pressure with a wate...
example 2
[0032] (1) In a 500ml three-neck flask, add 182g of trichloroacetyl chloride and 300mL of cyclohexane, and cool down to -5°C to 0°C in an ice-salt bath. Slowly feed 120g of vinyl methyl ether, after ventilation is complete, stir and react at room temperature for 12 hours, take a sample to control, trichloroacetyl chloride is basically reacted completely, and the water pump depressurizes to distill out cyclohexane and excess vinyl methyl ether, then, the oil pump reduces 181gBJ01 was obtained by pressure distillation, and the yield was 81% based on trichloroacetyl chloride.
[0033] (2) In a 1000ml three-necked flask, add 181g BJ01 and 250g anhydrous methanol, cool down to 0°C-10°C in an ice-salt bath, add 20g anhydrous sodium carbonate, stir, heat to 20°C-30°C, and keep warm for 12 Hour. After the reaction is complete, cool down to room temperature, filter with suction to remove solid salts, distill the filtrate under reduced pressure with a water pump to remove methanol, and...
example 3
[0036] (1) In a 500ml three-neck flask, add 182g of trichloroacetyl chloride and 300mL of dichloromethane, and cool down to -5°C to 0°C in an ice-salt bath. Slowly feed 120g of vinyl methyl ether, after ventilation is complete, stir and react at room temperature for 12 hours, take a sample and control, trichloroacetyl chloride is basically reacted completely, dichloroethane and excess vinyl methyl ether are distilled out under reduced pressure by the water pump, and then, the oil pump Distilled under reduced pressure to obtain 188g of BJ01, based on trichloroacetyl chloride, the yield was 84.1%.
[0037] (2) Add 188g of BJ01 and 250g of anhydrous methanol to a 1000ml three-neck flask, cool down to 0°C-10°C in an ice-salt bath, add 20g of anhydrous potassium carbonate, stir, heat to 20°C-30°C, and keep warm for 12 Hour. After the reaction is complete, cool down to room temperature, filter with suction to remove solid salts, distill the filtrate under reduced pressure with a wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com