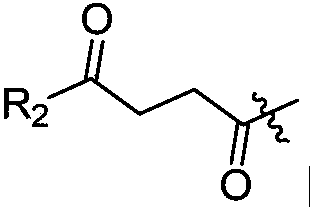
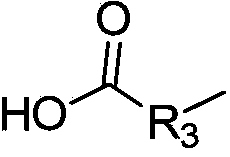
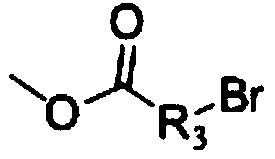
10-hydroxyevodiamine type anti-tumor compounds as well as preparation method and application thereof
A technology of hydroxyevodiamine and evodiamine, which is applied in the field of medicine, can solve the problems of poor water solubility and high toxicity, and achieve the effect of enhanced solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 110
[0120] The synthesis of embodiment 110-hydroxy evodiamine
[0121] 1. Preparation of N-formyl-5-methoxytryptamine
[0122] In a 50mL three-necked flask, add 4g (21mmol) 5-methoxytryptamine and 13g ethyl formate, and reflux at 80°C for 6 hours. Appeared slowly, 4.5g of the product was obtained by suction filtration, and the yield was 98.3%.
[0123] 2. Preparation of 3,4-dihydro-5-methoxy-β-carboline
[0124] In a 100mL three-necked flask, add 50mL of dichloromethane, under stirring conditions, add 4.0g (18mmol) of N-formyl-5-methoxytryptamine, cool to about 5°C in an ice-water bath, then slowly add 5.0mL Phosphorus oxychloride, reacted under ice bath for 2 hours, and then reacted at room temperature for 2 hours. After the reaction, dichloromethane and unreacted phosphorus oxychloride were recovered by distillation under reduced pressure, and the residual solid was separated with 120mL10% aqueous acetic acid solution. Three extractions. The combined extracts were adjusted t...
Embodiment 210
[0129] Synthesis of Example 210-((3-(tert-butoxy-2-((tert-butoxycarbonyl)amino)propionyl)oxy)evodiamine (L209)
[0130] In a 100mL round bottom flask, add (3-(tert-butoxy-2-((tert-butoxycarbonyl)amino)propionic acid 0.49g, EDC0.36g, DMAP0.23g, dissolve in 40mL DCM, stir at room temperature for 30min , Add 10-hydroxyevodiamine 0.50g (1.57mmol) again, react overnight, point plate monitoring.Reaction completes, directly evaporates to dryness solvent and mixes sample, column chromatography purification, eluent is sherwood oil: ethyl acetate=3: 1. Obtain 0.80 g of 10-((3-(tert-butoxy-2-((tert-butoxycarbonyl)amino)propionyl)oxy)evodiamine with a yield of 90.74%.
Embodiment 310
[0131] Example 31 Synthesis of 0-((1-(tert-butoxycarbonyl)pyrrolidine-2-carbonyl)oxy)evodiamine (L215)
[0132] Referring to Example 2, N-tert-butoxycarbonylproline was reacted with 10-hydroxyevodiamine to obtain 10-((1-(tert-butoxycarbonyl)pyrrolidine-2-carbonyl)oxy)evodiamine 0.74 g, the total yield is 92.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com