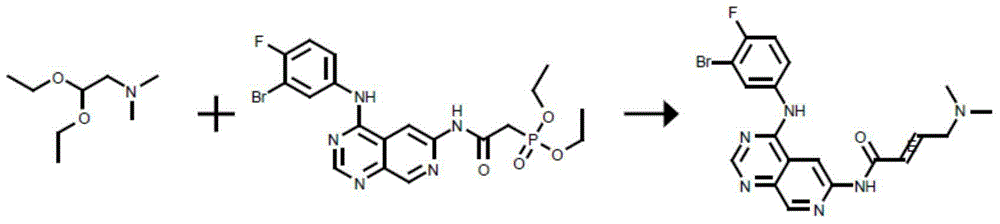
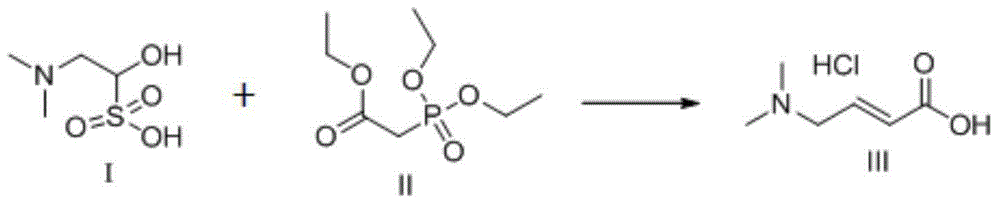
Trans-4-dimethylamino crotonic acid hydrochloride preparation method
A technology of dimethylaminocroton hydrochloride and dimethylamino group, which is applied in the field of preparation of crotonic acid derivatives, can solve problems such as inability to obtain products, affect stereoselectivity, and unsatisfactory reaction yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] In order to facilitate the understanding of the technical solutions of the present invention, the following will be introduced in conjunction with specific implementation manners.
[0016]
[0017] Operating process:
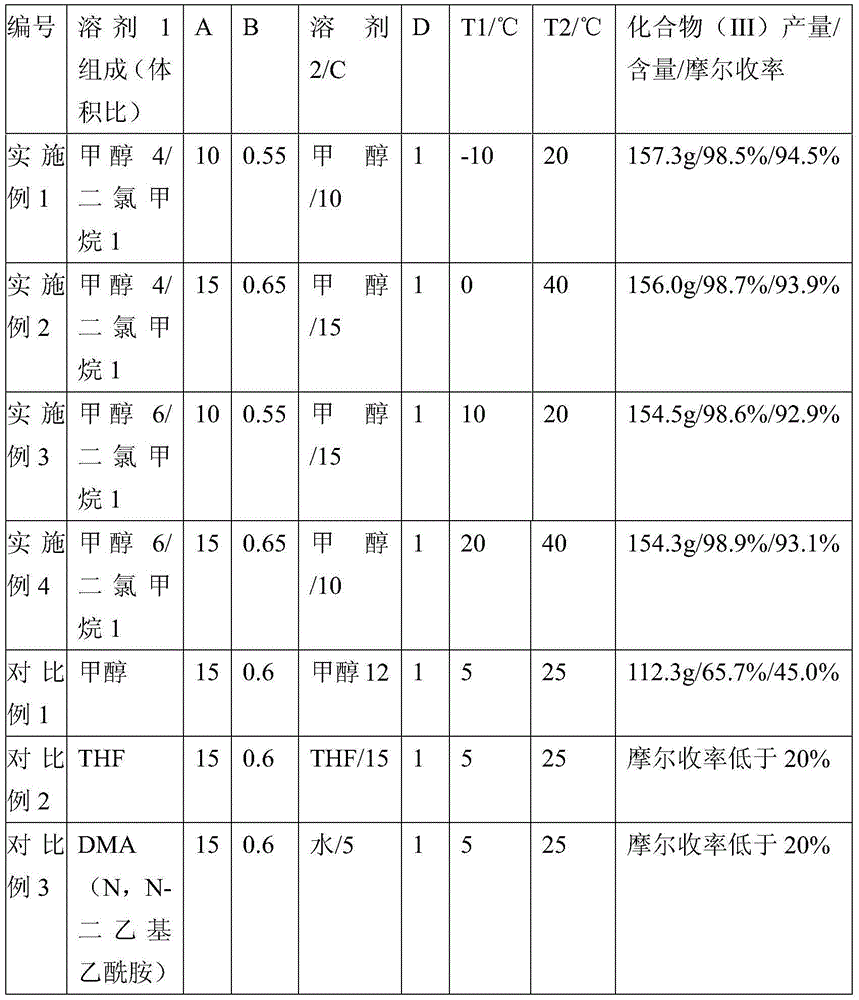
[0018] 1) Dissolving triethyl phosphonoacetate (II) in solvent 1, the weight to volume ratio of triethyl phosphonoacetate to solvent 1 is 1:A;
[0019] 2) NaOH is added to solvent 2 and stirred evenly, the weight ratio of NaOH to triethyl phosphonoacetate (II) is B, and the weight-to-volume ratio of NaOH to solvent 2 is 1:C, the NaOH dispersed in solvent 2 Slowly add the triethyl phosphonoacetate (II) dissolved in solvent 1 obtained in step 1), and stir for 1 h after the addition is complete to obtain the reaction solution (3), and the reaction temperature is controlled at T1°C;
[0020] 3) Dissolve N,N-dimethylaminoacetaldehyde bisulfite (I) in water and slowly add it dropwise to the reaction solution (3). Descending to obtain reaction solution (4),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com