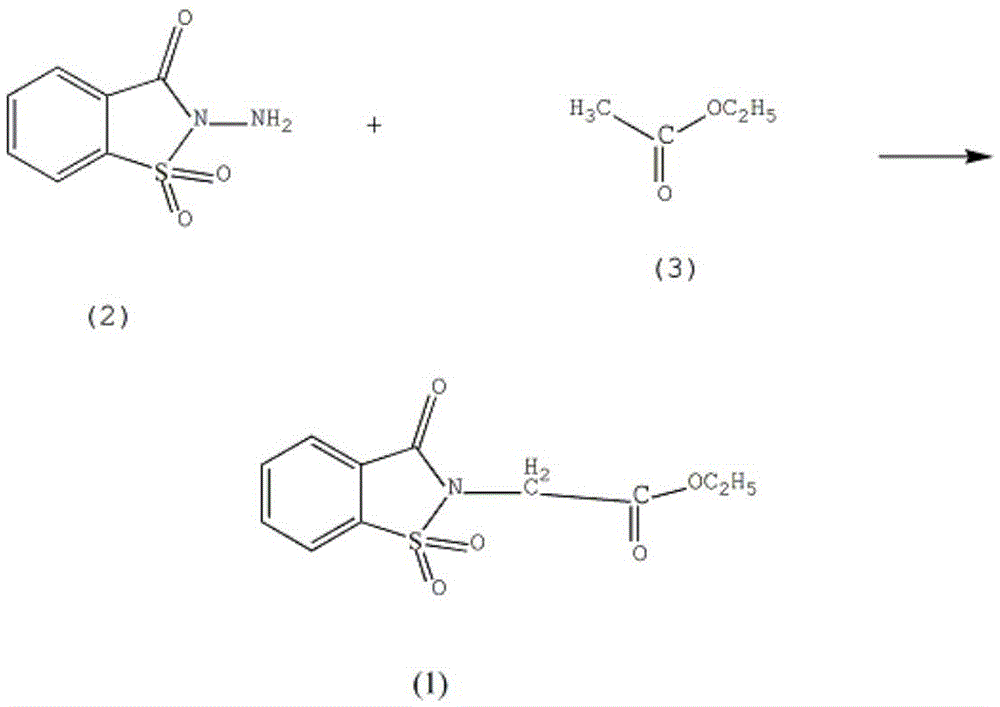
Synthetic method of piroxicam drug intermediate 3-oxo-1,2-benzisothiazole-1,1-dioxo-2-ethyl acetate
A technology of benzisothiazole and piroxicam, applied in the direction of organic chemistry, can solve problems such as many adverse reactions in the gastrointestinal tract, and achieve the effects of reducing reaction temperature and reaction time, reducing intermediate links and improving reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0012] In the reaction vessel equipped with a stirrer, reflux condenser, dropping funnel, and thermometer, add 2.16mol of saccharinamine (2), 2.1L of nitromethane, control the stirring speed at 160rpm, raise the solution temperature to 110°C, Slowly add 2.3 mol of ethyl acetate (3), continue to stir for 11 hours after the addition is complete, steam most of the nitromethane, pour the reactant into a 15% potassium chloride solution by mass fraction, and filter with suction to obtain a white solid. 900ml of 80% acrylonitrile was recrystallized, the temperature of the solution was lowered to 3°C, allowed to stand for 30 hours, suction filtered, washed with 90% toluene, and dehydrated with anhydrous calcium sulfate to obtain 3-oxo-1,2-benzene Ethyl isothiazole-1,1-dioxo-2-acetate 418.35g, yield 72%.
example 2
[0014] In the reaction vessel equipped with a stirrer, reflux condenser, dropping funnel, and thermometer, add 2.16mol of saccharinamine (2), 2.2L of nitromethane, control the stirring speed at 170rpm, raise the solution temperature to 112°C, Slowly add 2.4 mol of ethyl acetate (3), continue to stir for 112 hours after the addition is complete, steam most of the nitromethane, pour the reactant into a 17% potassium chloride solution by mass fraction, and filter with suction to obtain a white solid. 900ml of 82% acrylonitrile was recrystallized, lowered the temperature of the solution to 4°C, allowed to stand for 32 hours, filtered with suction, washed with 92% toluene, and dehydrated with activated alumina to obtain 3-oxo-1,2-benzo Isothiazole-1,1-dioxo-2-ethyl acetate 435.78g, yield 75%.
example 3
[0016] In the reaction vessel equipped with a stirrer, reflux condenser, dropping funnel, and thermometer, add 2.16mol of saccharinamine (2), 2.3L of nitromethane, control the stirring speed at 190rpm, raise the solution temperature to 115°C, Slowly add 2.5 mol of ethyl acetate (3), continue to stir for 13 hours after the addition is complete, evaporate most of the nitromethane, pour the reactant into a 20% potassium chloride solution by mass fraction, and filter with suction to obtain a white solid. 900ml of 85% acrylonitrile was recrystallized, the temperature of the solution was lowered to 5°C, allowed to stand for 36 hours, filtered with suction, washed with 95% toluene, and dehydrated with anhydrous calcium sulfate to obtain 3-oxo-1,2-benzene Ethyl isothiazole-1,1-dioxo-2-acetate 470.64g, yield 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com