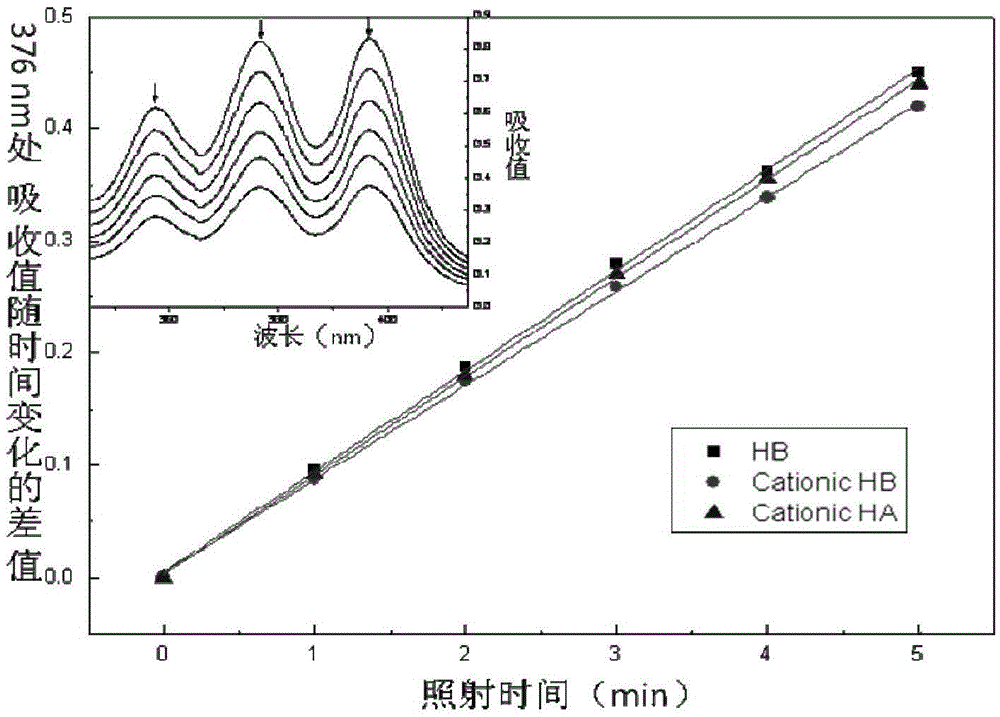
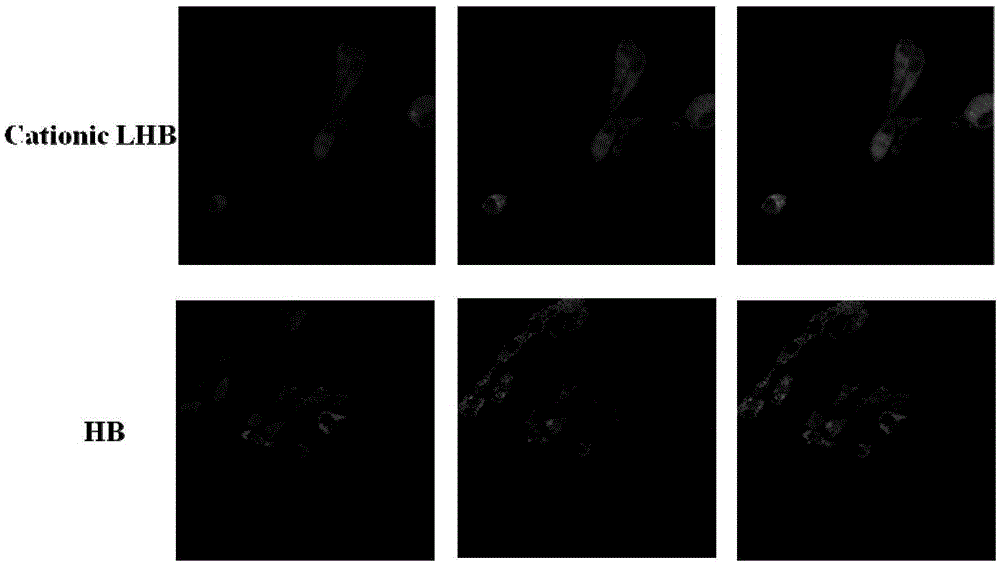
Hypocrellin cationic liposome preparation and preparation method and application thereof
A technology of cationic lipid and oleocanthin, which is applied in liposome delivery, pharmaceutical formulation, photodynamic therapy, etc., can solve the problem of not being able to accurately target neovascular endothelium, and not being able to well target lesion malformations. New blood vessels and other problems, to achieve the effect of high dosage form stability, simple preparation process and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, prepare hypocrellin cationic liposome preparation
[0048] Hypocretin cationic liposome preparation is prepared according to the following steps:
[0049] (1) Dissolve 50 mg of Hypocretin B, 900 mg of DOTAP, 950 mg of DOPC, 110 mg of cholesterol and 25 mg of STPP in 100 mL (150 g) of chloroform, then place it in a rotary evaporator, and rotate it under a pressure of 8 mbar for 2 hours to prepare a thin film.
[0050] (2) Add 50 mL (50 g) of PBS solution to the film prepared in the above step (1), and shake it at 200 rpm for 4 hours to form multilocular liposomes.
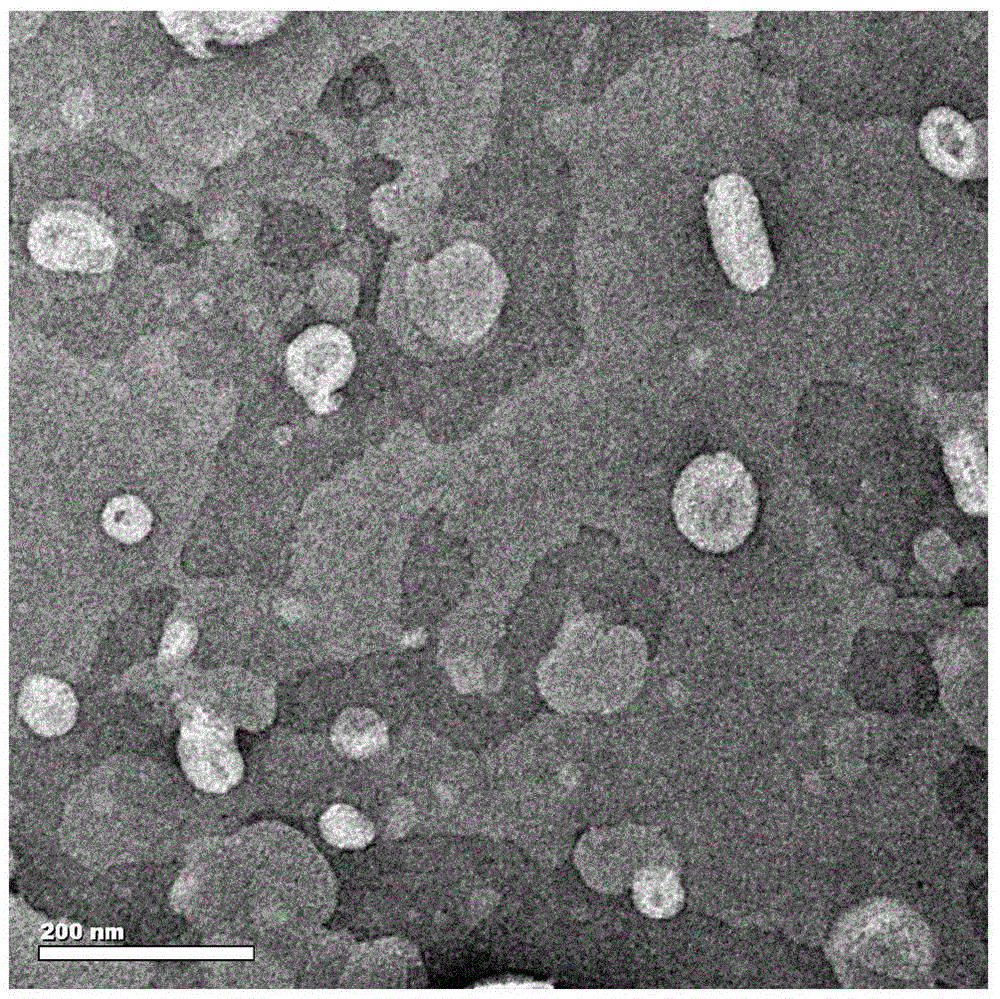
[0051] (3) Place the multilamellar liposome prepared in the above step (2) at 0° C. and ultrasonicate for 45 minutes under the condition of ultrasonic power of 80 W to obtain unilamellar liposomes with a particle size distribution of 57-119 nm.
[0052] (4) Chromatographically separate the unilamellar liposomes prepared in the above step (3) with SephadexG-15 dextran gel (eluent: PBS, pH=7.2~7....
Embodiment 2
[0053] Embodiment 2, prepare hypocrellin cationic liposome preparation
[0054] Hypocretin cationic liposome preparation is prepared according to the following steps:
[0055] (1) Dissolve Hypocretin B 100mg, DPTAP 1850mg, DOPC 1850mg, Cholesterol 220mg and STPP 50mg in 200mL (300g) of chloroform, then place it in a rotary evaporator, and spin evaporate it under 10mbar pressure for 3 hours to prepare a thin film.
[0056] (2) 100 mL (100 g) of PBS solution was added to the film prepared in the above step (1), and shaken at 200 rpm for 4 h to form multilocular liposomes.
[0057] (3) Place the multilamellar liposome prepared in the above step (2) at 4° C., and ultrasonicate for 0.5 h under the condition of ultrasonic power of 120 W, to obtain unilamellar liposomes with a particle size distribution of 55-122 nm.
[0058] (4) Chromatographically separate the unilamellar liposomes prepared in the above step (3) with SephadexG-15 dextran gel (eluent: pH=7.2~7.4, concentration is 0...
Embodiment 3
[0059] Embodiment 3, preparation hypocretin cationic liposome preparation
[0060] Hypocretin cationic liposome preparation is prepared according to the following steps:
[0061] (1) Dissolve Hypocretin B 150mg, DDAB2670mg, DOPC2910mg, Cholesterol 330mg and STPP75mg in 300mL (398g) of dichloromethane, then place it in a rotary evaporator, and rotate it under a pressure of 10mbar for 4 hours to prepare film.
[0062] (2) 150 mL (150 g) of PBS solution was added to the film prepared in the above step (1), and shaken at 200 rpm for 5 h to form multilocular liposomes.
[0063] (3) Put the multilamellar liposome prepared in the above step (2) at 15° C. and ultrasonicate for 1 h under the condition of ultrasonic power of 200 W to obtain unilamellar liposomes with a particle size distribution of 50-120 nm.
[0064] (4) SephadexG-15 dextran gel is used to carry out chromatographic separation of the single-chamber liposomes prepared above, remove the unencapsulated STPP in the liposo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com