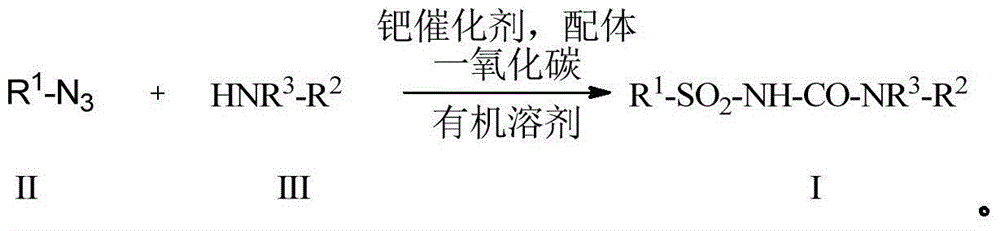
Preparation method for alkyl/benzyl/aryl urea compounds through heterogeneous-phase catalysis
A compound, cycloalkylalkyl technology, applied in the field of preparation of alkyl/benzyl/aryl urea compounds, can solve the problems of poor product universality, high price, high toxicity, etc., and achieve a wide range of substrates Adaptability, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
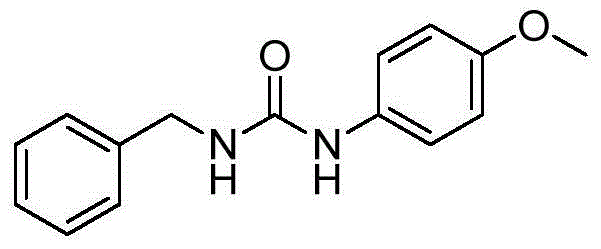
[0063] The preparation of embodiment 1N-benzyl-N'-(4-methoxyphenyl) urea
[0064] Chemical name: N-Benzyl-N'-(4-methoxyphenyl)urea
[0065] Molecular formula: C 15 h 16 N 2 o 2
[0066] CAS registration number: 126679-87-6
[0067]
[0068] Steps:
[0069] Add palladium / carbon (21mg, 0.02mmol), XPhos (19mg, 0.04mmol), p-methoxyaniline (59mg, 0.48mmol), toluene (4ml) successively to the 25ml reaction bottle, the system is vacuumed to 20mmHg, and then filled with Inject CO gas to normal pressure, repeat this 3 times, then add benzyl azide (53 mg, 0.40 mmol) using a syringe, and place the system at 60 o After vigorously stirring at C for 12 hours, the solvent was concentrated under reduced pressure, and the residue was separated and purified by column chromatography (petroleum ether: ethyl acetate (volume ratio) = 3:1) to obtain 94 mg of a white solid product with a yield of 91%. The characterization data of the obtained compound are as follows:
[0070] MSm / z(ESI):25...
Embodiment 2-41
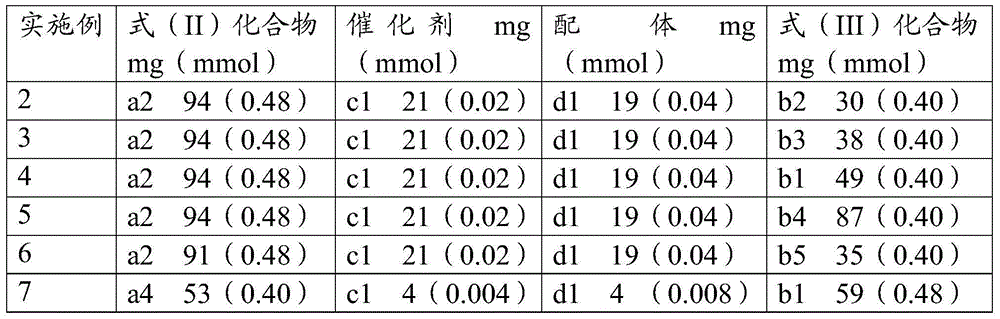
[0073] Examples 2-41 were prepared by the same method as Example 1, and the specific raw material ratios are shown in Table 2.
[0074] The temperature of reaction of table 2 embodiment 2-41 and concrete raw material proportioning
[0075]
[0076]
[0077]
[0078] Table 3 Example 2-41 product information and characterization
[0079]
[0080]
[0081]
[0082]
[0083]
[0084]
[0085]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com