Method for preparing aniline-2-sulfonic acid
A technology for o-aminobenzenesulfonic acid and aniline, which is applied in the field of preparing o-aminobenzenesulfonic acid by catalytic sulfonation of aniline, can solve the problems of no development prospect, large three wastes, low yield and the like, and achieves low cost, less three wastes, and a process route. short effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
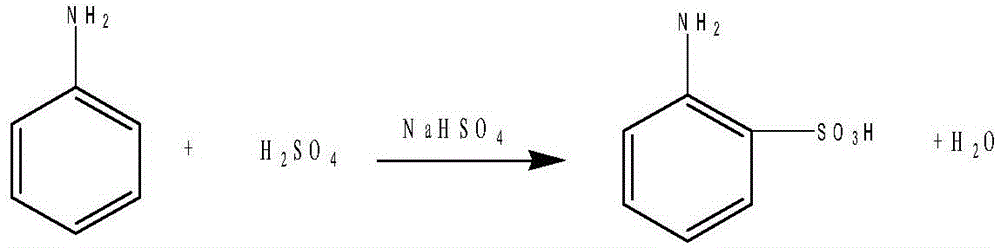
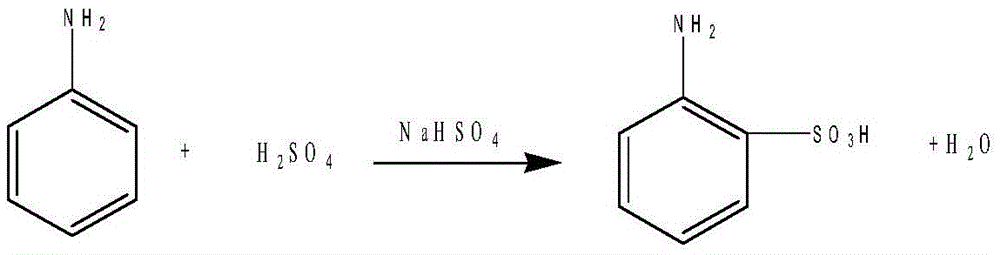
[0018] The synthesis of anthranilic acid, reaction formula is as follows:
[0019]
[0020] Add 94 grams of aniline, 100 grams of concentrated sulfuric acid (concentrated sulfuric acid with a concentration of 98%), and 120 grams of sodium bisulfate into the disperser in turn, disperse for 1 hour, then pour the uniformly dispersed material into a baking tray, and put it into a vacuum drying In the box, heat to 200°C, keep warm for 18 hours, pour the material into 600ml of water, add 1 gram of activated carbon, heat to 80°C for decolorization, filter out the activated carbon after decolorization, stir, cool to room temperature, crystallize, filter to obtain white Solid 168 grams, content: 99%. The filtrate was applied to the next batch.
Embodiment 2
[0022] Synthesis of anthranilic acid
[0023] Add 94 grams of aniline, 100 grams of concentrated sulfuric acid, and 136 grams of potassium bisulfate into the disperser in sequence, and disperse for 1 hour, then pour the uniformly dispersed materials into a baking tray, put them in a vacuum drying oven, heat to 210°C, and keep warm After 18 hours, the material was poured into the water (600ml) obtained in Example 1, and 1 gram of activated carbon was added, heated to 80°C for decolorization, and the activated carbon was filtered after decolorization, stirred, crystallized, and filtered to obtain 170 grams of white solid, content : 99%. The filtrate was applied to the next batch.
Embodiment 3
[0025] Synthesis of anthranilic acid
[0026] Add 94 grams of aniline, 100 grams of concentrated sulfuric acid, and 144 grams of sodium bisulfate into the disperser in sequence, and disperse for 1 hour, then pour the uniformly dispersed materials into a baking tray, put them in a vacuum drying oven, heat to 210°C, and keep warm After 18 hours, the material was poured into the water (600ml) obtained in Example 2, and 1 gram of activated carbon was added, heated to 80° C. for decolorization, and the activated carbon was filtered after decolorization, stirred, crystallized, and filtered to obtain 172 grams of white solid, content : 99%. The filtrate is evaporated to recover sodium bisulfate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com