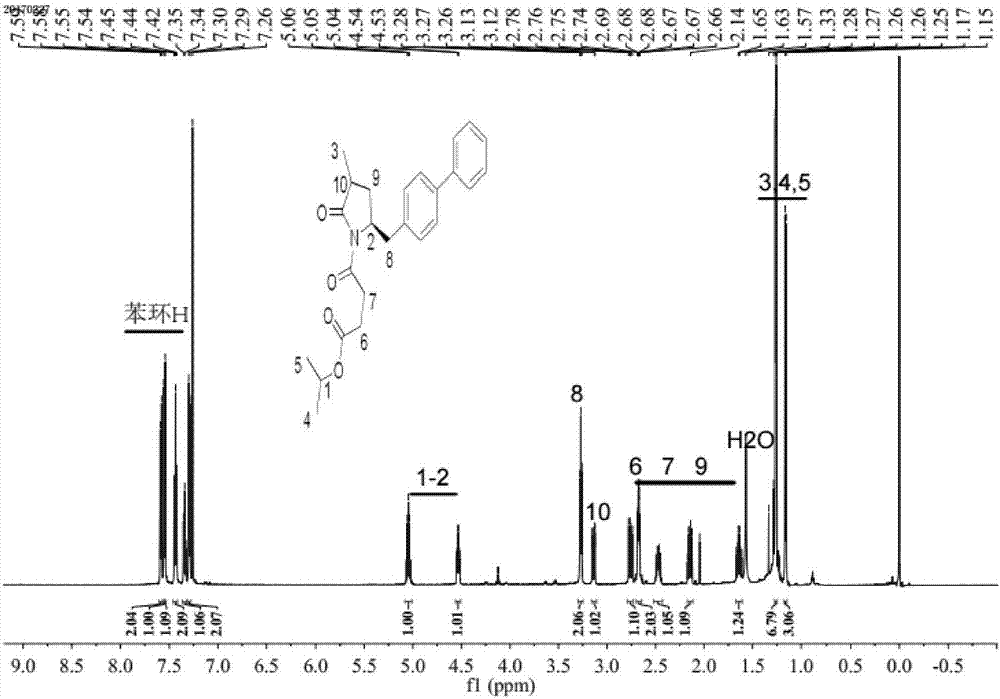
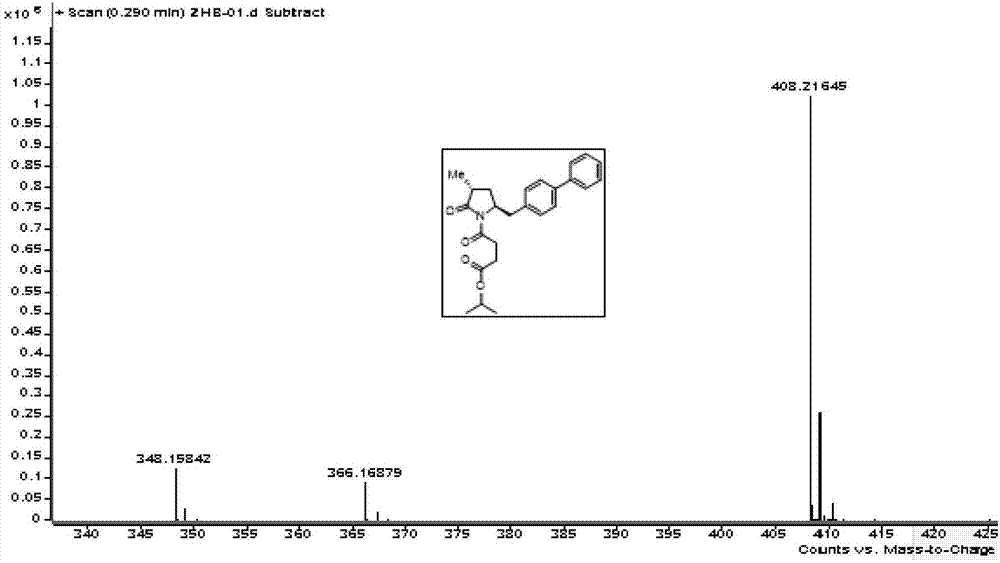
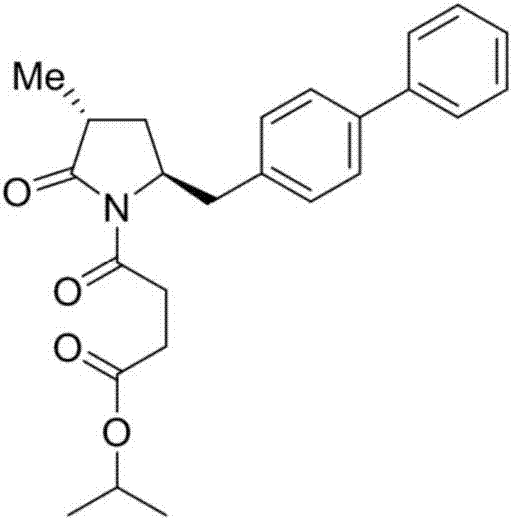
Method for preparing LCZ696 impurity reference substance
An impurity reference substance and compound technology, applied in the direction of organic chemistry, etc., can solve the problems of LCZ696 drug impurity synthesis report and other problems that have not yet appeared, and achieve the effects of economical and environmentally friendly process route, reduction of use, and avoidance of use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0021] The drug source used in the preparation method of the embodiment of the present invention is shown in Table 1.
[0022] Table 1
[0023]
[0024]
Embodiment 1
[0025] The preparation of embodiment 1LCZ696 impurity reference substance
[0026] (S1) compound shown in preparation formula I
[0027] 1g (2R,4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid, 15ml ethanol, 0.25ml thionyl chloride (0.0034mol ) was added into the reaction bottle, and the temperature was raised to 78° C. for reaction for 4 to 9 hours. The reaction solution was a colorless transparent solution, which was concentrated to remove the solvent to obtain 0.75 g of a white solid, which was the compound represented by formula I, with a yield of 88%.
[0028]
[0029] (S2) compound shown in preparation formula II
[0030] Add 2g of the compound represented by formula I (0.0064mol), 30ml of ethyl acetate, and 1g of triethylamine (0.0099mol) into the reaction flask, and react at 77°C. The reaction solution is a colorless transparent liquid, and the reaction is stopped after 3 to 5 hours. Reaction, remove solvent to obtain 1.2g white solid, ...
Embodiment 2
[0041] The preparation of embodiment 2LCZ696 impurity reference substance
[0042] The other steps of this example are the same as Example 1, except that the preparation conditions of the compound represented by formula I are different. The steps of the compound shown in the preparation formula I are as follows:
[0043] 1g (2R,4S)-5-(biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid (0.0026mol), 15ml ethanol, 0.19ml chlorinated Sulfone (0.0026mol) was added into the reaction flask, and the temperature was raised to 78° C. for 7 hours of reaction. The reaction solution was a colorless transparent solution, which was concentrated to remove the solvent to obtain 0.6 g of a white solid, namely the compound represented by formula I, with a yield of 70%.
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com