Preparing and detecting method for bacterium phosphopantetheinyl transferase antibody
A bacterial phosphopantetheinyl, phosphopantetheinyl technology, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The expression and purification of bacterial phosphopantetheinyl transferase protein is carried out according to the following steps:
[0028] (1) Cloning enzyme protein gene sequence
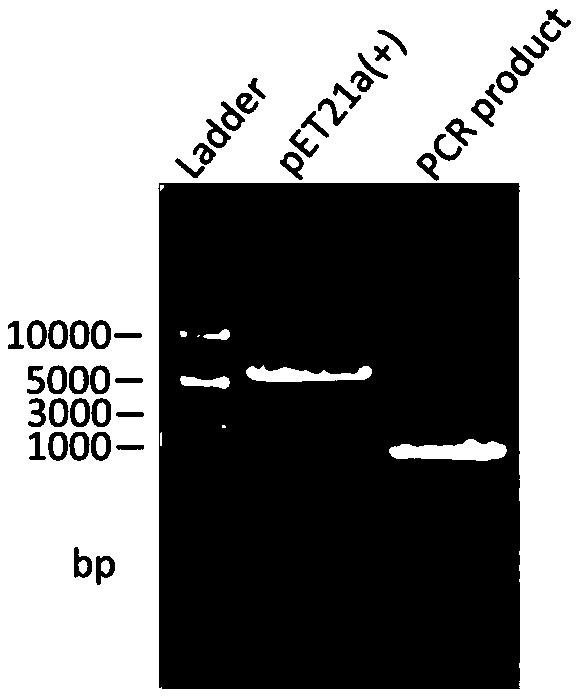
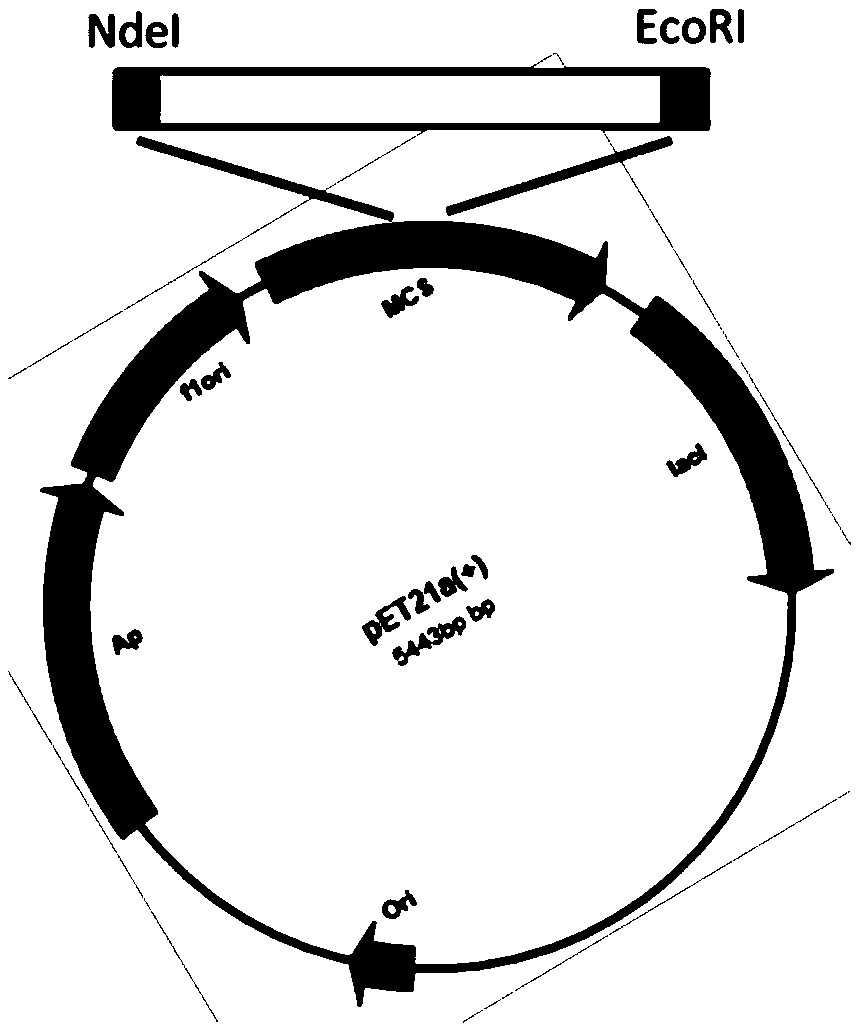
[0029] Primers F1 and R1 were designed by comparing the homologous gene sequences of phosphopantetheinyl transferases of strains such as Shewanellasp.SC2A and Shewanellasp.Ac10 provided by GnenBank (see Table 1). The amplification uses the genome of Shewanellasp.Ac10 as a template, and the amplification reaction system is as follows: 5 μl of 10×PCR reaction buffer, 25mmol / LMgSO 4 2μl, 5μl of 10mmoldNTP, 1μl of each 10nmol / L primer, 80ng of genomic DNA template, 1μl of KoD-Plus DNA polymerase, and distilled deionized water to 50μl. The reaction conditions were: pre-denaturation at 95°C for 5 min; 30 cycles at 95°C for 30 s, 55°C for 30 s, and 68°C for 1.30 min; extension at 68°C for 10 min; 15°C for 10 min. The PCR amplified products were subjected to 1% agarose gel electrophoresis, rec...
Embodiment 2
[0039] The method for preparing an antibody by bacterial phosphopantetheinyl transferase comprises the following steps:
[0040] (1) preparing higher-purity phosphopantetheinyl transferase;
[0041] (2) Using higher-purity phosphopantetheinyl transferase as antigenic protein, adopting conventional polyclonal antibody preparation method to prepare antiserum;
[0042] (3) The antiserum is subjected to antibody purification treatment.
[0043] In step (1), the preparation method of higher-purity phosphopantetheinyl transferase is to take the purified phosphopantetheinyl transferase for polyacrylamide gel electrophoresis, and then perform gel electrophoresis. Staining, after staining, cut out the phosphopantetheinyl transferase protein in polyacrylamide gel, place the cut gel piece in a dialysis bag, and conduct further electrophoresis in an electrophoresis tank to separate out the protein completely. And remove the gel block, concentrate to obtain higher purity antigenic protei...
Embodiment 3
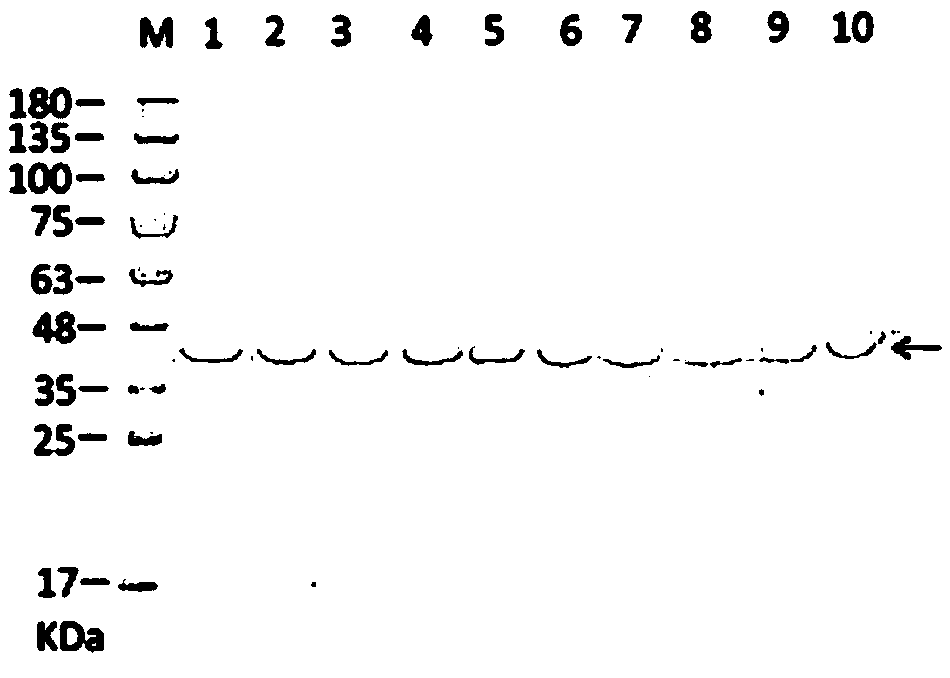
[0047] The detection methods of polyclonal antibody derived from rabbits for phosphopantetheinyl transferase include determination of purified antibody concentration, ELISA titer determination, and Western Blotting antibody specificity determination.
[0048] The method for determining the concentration of the purified antibody comprises the following steps:
[0049] (1) Preparation of CBB staining solution: According to the number of samples, dilute 5×CBB staining solution and mix well.
[0050] (2) Take an appropriate amount of BSA standard protein as needed, prepare standard samples with final concentrations of 1mg / ml, 0.75mg / ml, 0.5mg / ml, 0.25mg / ml and 0.125mg / ml, and mix well. The standard sample uses PBS as solvent.
[0051] (3) Take a microtiter plate and add reagents according to Table 2.
[0052] Table 2 Reagents for ELISA plate
[0053] Hole number
A
B
C
D
E
F
Standard concentration (mg / ml)
0
0.125
0.25
0.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com