Method for efficient synthesis of difructose anhydride III
A double fructose anhydride, high-efficiency technology, applied in the field of functional food bioprocessing, can solve the problems of uneconomical and high prices, and achieve the effects of low production cost, low energy consumption and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Dissolve the sucrose substrate completely with deionized water, control its concentration to 400g / L, adjust the pH value to 5.5, control the temperature at 20°C, add inulin sucrase at an amount of 10U / g sucrose, and react at a constant temperature for 30 minutes;
[0032] Raise the temperature of the reaction system to 55°C, and then add inulin fructotransferase to it, the concentration of the added inulin fructotransferase is 5U / g, the pH value is still controlled at 5.5, and the reaction is carried out at constant temperature for 12 hours;
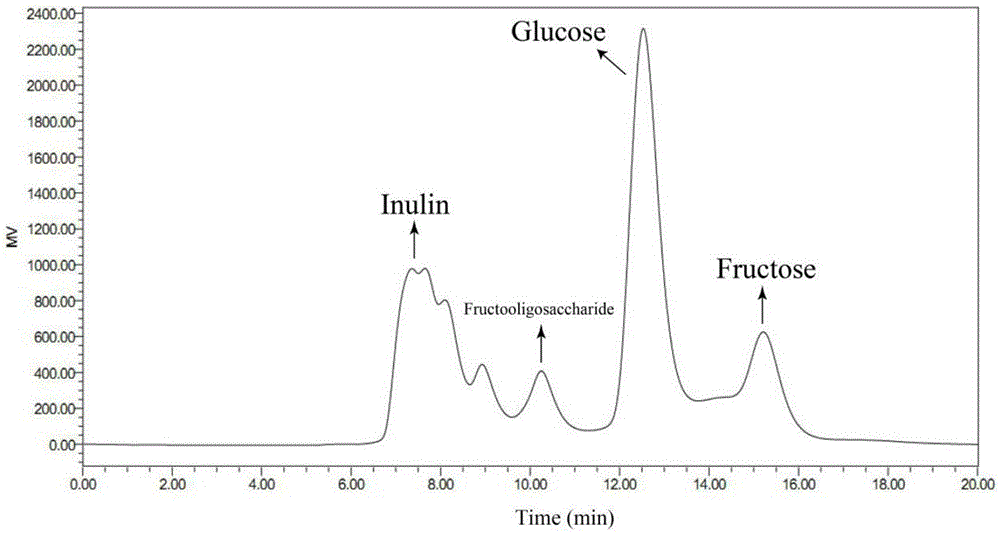
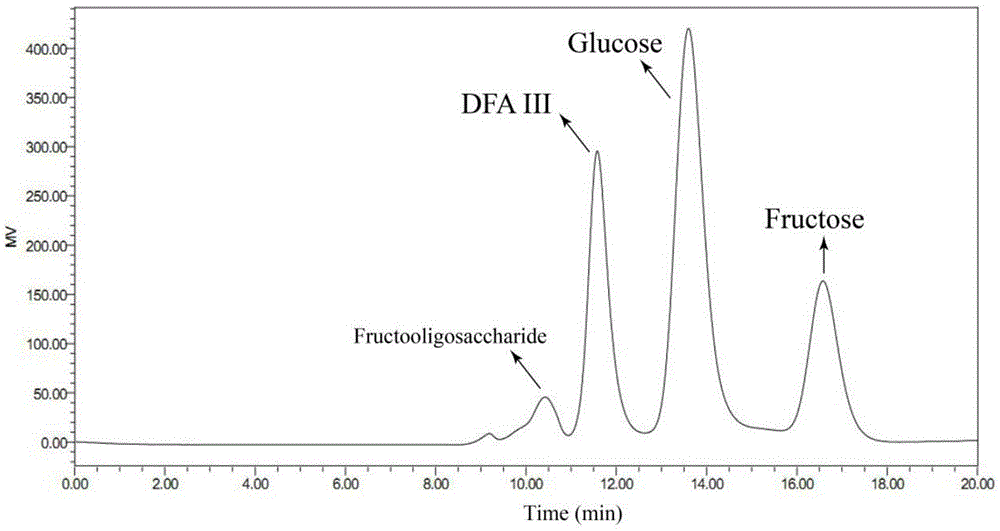
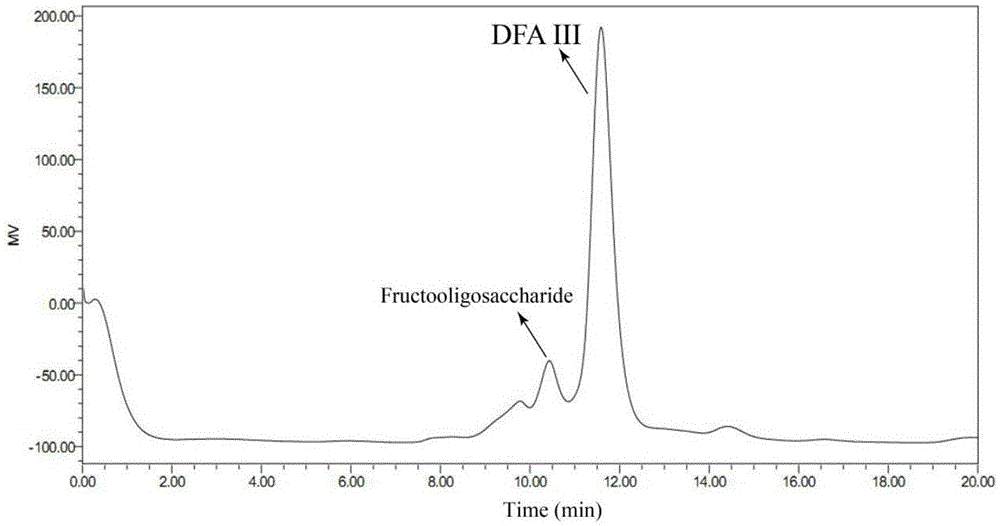
[0033] The solution after the reaction was taken and centrifuged, the supernatant was filtered with a microporous membrane (0.22), and the filtrate was analyzed by HPLC equipped with a differential refraction display. HPLC conditions are: Sugarpak1, 6.5mmid×300mm calcium type cation exchange column, pure water as mobile phase, column temperature is 85°C, and tassel is 0.4ml / min. The injection volume was 10ul, and the concentration...
Embodiment 2
[0035] Dissolve the sucrose substrate completely with deionized water, control the concentration to 300g / L, adjust the pH value to 5.0, control the temperature at 22°C, add inulin sucrase at an amount of 15U / g sucrose, and react at a constant temperature for 60 minutes;
[0036] Raise the temperature of the reaction system to 60°C, and then add inulin fructosyltransferase to it, the concentration of the added inulin fructosyltransferase is 10U / g, the pH value is still controlled at 5.0, and the constant temperature reaction is 6h; the obtained double The conversion rate of fructose III reached 48%.
Embodiment 3
[0038] Dissolve the sucrose substrate completely with deionized water, control the concentration to 500g / L, adjust the pH value to 6.0, control the temperature at 30°C, add inulin sucrase, the addition amount is 5U / g sucrose, and react at constant temperature for 60min;
[0039] Raise the temperature of the reaction system to 50°C, and then add inulin fructosyltransferase to it, the concentration of the added inulin fructosyltransferase is 20U / g, the pH value is still controlled at 6.0, and the constant temperature reaction is 20h; the obtained double The conversion rate of fructose III reached 43%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com