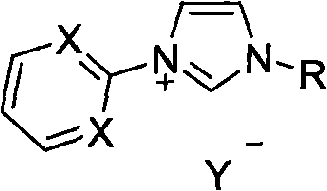
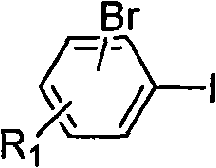
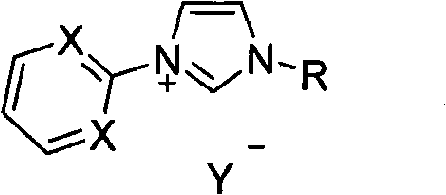
Method for preparing asymmetric substituted aryl compound or terphenyl derivative by one-pot tandem reaction of functionalized imidazole salt and palladium salt
A series reaction, aryl dihalide technology, applied in the field of one-pot series reaction catalyzed by functionalized imidazolium salt and palladium salt to prepare asymmetric substituted aryl compounds or terphenyl derivatives, can solve the problem of less application research and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] Preparation of (E)-butyl 3-(biphenyl-4-yl)acrylate. The reaction formula is as follows:
[0127]
[0128] The specific preparation method is: add 4-bromoiodobenzene (282mg, 1.0mmol), 5mL DMF solvent, L·HCl (6mg, 2.0mol%), Pd(OAc) in the Schlenk reaction tube successively 2 (2 mg, 1.0 mol%), NaOAc (100 mg, 1.2 mmol), n-butyl acrylate (134 mg, 1.05 mmol). Then the reaction solution was heated to 120° C. for 2 hours and then cooled to room temperature. Phenylboronic acid (183 mg, 1.5 mmol) and Cs were added directly 2 CO 3 (400mg, 1.2mmol) and continue to heat up to 80°C for 3 hours. After the reaction solution was cooled to room temperature, it was poured into 20 mL of water, and the 2 Cl 2 (3 x 20 mL) extraction. The combined organic layers were washed with water (2×20 mL) and washed with anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (ethyl acetate / petroleum ether=5 / 95) ...
Embodiment 2
[0130] Preparation of (E)-ethyl 3-(4'-chlorob iphenyl-4-yl)acrylate. The reaction formula is as follows:
[0131]
[0132] The specific preparation method is: add 4-bromoiodobenzene (282mg, 1.0mmol), 5mL DMAc solvent, L HCl (6mg, 2.0mol%), Pd(OAc) in the Schlenk reaction tube successively 2 (2 mg, 1.0 mol%), NaOAc (100 mg, 1.2 mmol), ethyl acrylate (105 mg, 1.05 mmol). Then the reaction solution was heated to 120° C. for 1 hour and then cooled to room temperature. 4-Chlorophenylboronic acid (234 mg, 1.5 mmol) and Cs were added directly 2 CO 3 (400mg, 1.2mmol) and continue to heat up to 80°C for 3 hours. After the reaction solution was cooled to room temperature, it was poured into 20 mL of water, and the 2 Cl 2 (3 x 20 mL) extraction. The combined organic layers were washed with water (2×20 mL) and washed with anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (ethyl acetate / petrol...
Embodiment 3
[0134]Preparation of (E)-butyl 3-(4'-chlorobiphenyl-3-yl)acrylate. The reaction formula is as follows:
[0135]
[0136] The specific preparation method is: add 3-bromoiodobenzene (282mg, 1.0mmol), 5mL DMF solvent, L·HCl (3mg, 1.0mol%), Pd(OAc) in the Schlenk reaction tube successively 2 (4 mg, 2.0 mol%), NaOAc (162 mg, 2.0 mmol), n-butyl acrylate (134 mg, 1.05 mmol). Then the reaction solution was heated to 120° C. for 2 hours and then cooled to room temperature. 4-Chlorophenylboronic acid (234 mg, 1.5 mmol) and Cs were added directly 2 CO 3 (400mg, 1.2mmol) and continue to heat up to 80°C for 3 hours. After the reaction solution was cooled to room temperature, it was poured into 20 mL of water, and the 2 Cl 2 (3 x 20 mL) extraction. The combined organic layers were washed with water (2×20 mL) and washed with anhydrous MgSO 4 After drying, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (ethyl acetate / petrol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com