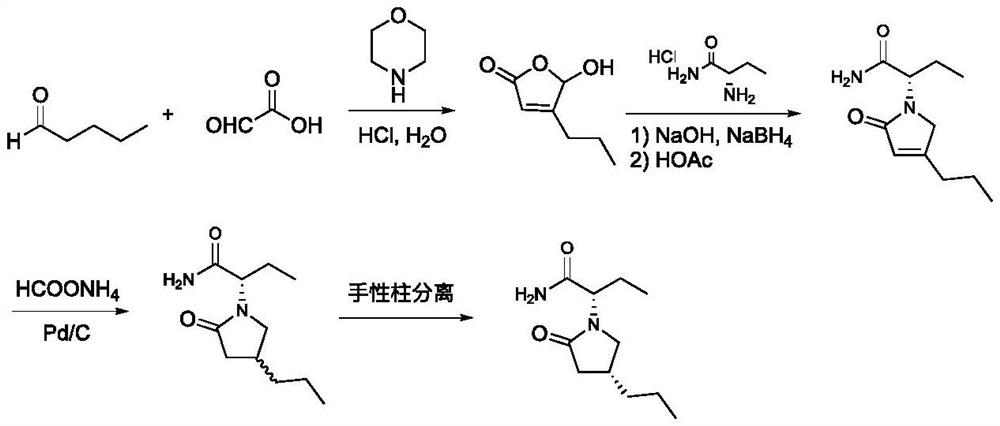
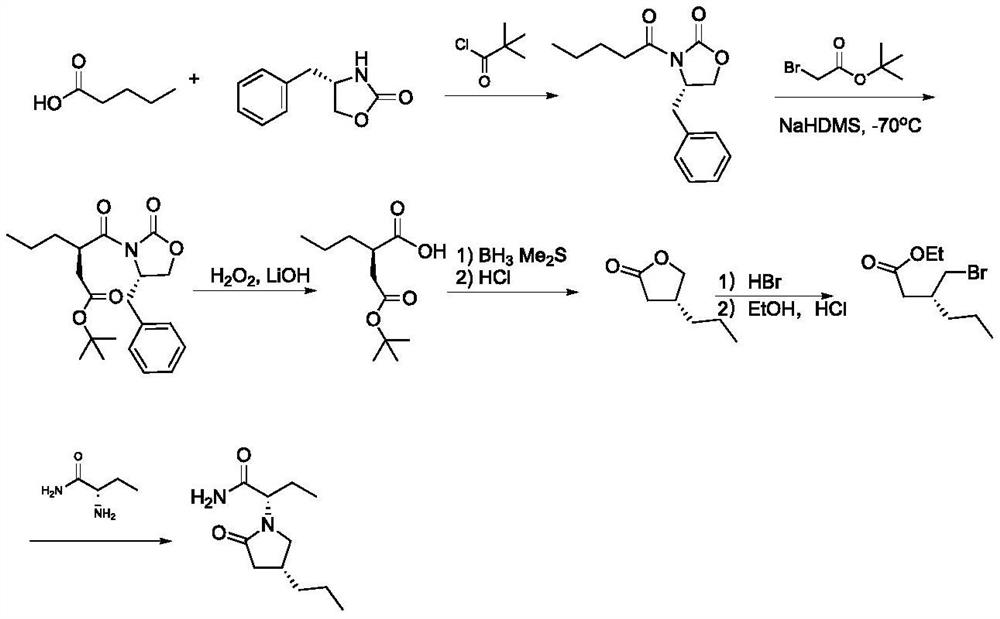
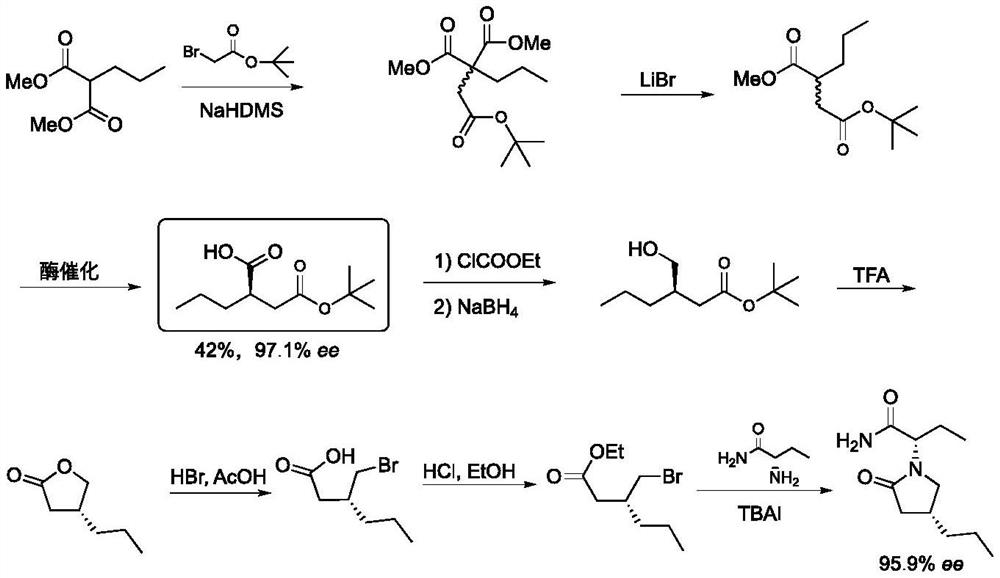
Baeyer-Villiger monooxygenase and application thereof in synthesis of briracetam
A monooxygenase, briracetam technology, applied in the application, oxidoreductase, enzyme and other directions, can solve the problems of explosion risk, difficult to meet medicinal requirements, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the preparation of 3-propyl cyclobutanone (compound 1)
[0063] The process route is as follows:
[0064]
[0065] Dissolve trichloroacetyl chloride (2.24mL, 20mmol) and phosphorus oxychloride (1.02mL, 11.0mmol) in ether (10mL), then slowly drop this solution into a solution containing 1-pentene (1.09mL, 10mmol) , diethyl ether (20mL) and zinc copper reagent (Zinc-Copper couple, CAS#: 53801-63-1, 1.96g, 30.0mmol) in a flask. Heated to 40°C and stirred for 2 hours, then naturally cooled to room temperature and stirred for 8 hours. Afterwards, the solution was filtered with diatomaceous earth, and 80 mL of n-hexane was added to the filtrate to precipitate zinc chloride salt. The clear solution was obtained by filtration, washed successively with water, saturated sodium bicarbonate solution and saturated brine, dried over anhydrous sodium sulfate, concentrated under reduced pressure, and evaporated to remove the solvent. The light yellow oily substance ...
Embodiment 2
[0067] Embodiment 2: the preparation of Baeyer-Villiger monooxygenase
[0068] 1.1 Acquisition of enzyme gene
[0069] Select CHMO from Acinetobacter sp. (CHMO Acineto ), for rational enzyme design and site-directed mutagenesis. According to the above monooxygenase sequence retrieved by NCBI, the wild-type CHMO from Acinetobacter sp. monooxygenase gene (CHMO Acineto Gene).
[0070] 2.2 Construction and transformation of enzyme gene
[0071] CHMO synthesized in step 1.1 Acineto The gene was inserted between the restriction enzyme sites Nde I and BamH I in the multiple cloning site region of the pEt-22b (+) plasmid to obtain wild-type CHMO Acineto gene plasmid. Primers were designed for each mutation site of the protein according to the amino acid sequence shown in sequence 1-5, and wild-type CHMO Acineto The plasmid of the gene is used as a template, and polymerase chain reaction (PCR) is used to perform site-directed mutation to obtain the plasmids of genes encoding Bae...
Embodiment 3
[0076] Example 3: Preparation of (R)-4-propyl-dihydrofuran-2-one (compound 2)
[0077] The process route is as follows:
[0078]
[0079] Add 100 mL of the whole cell buffer solution obtained in Example 2 and 100 mmol of cyclic ketone substrate into a 500 mL Erlenmeyer flask, react for 8 hours, break the cells with an ultrasonic cell disruptor, centrifuge, extract with ethyl acetate (100 mL*3), and combine In the organic phase, the extract was rotary evaporated to remove the solvent, and ethyl acetate containing 0.2 mg / mL dodecane was dissolved again. The extract was detected by gas chromatography for product yield and enantiomeric excess value. The results are shown in Table 1.
[0080] Table 1
[0081] Enzyme type Conversion rates(%) Product ee value (%) L143A / L244A (SEQ ID No: 1) 98.9 99.7 L143V / F277V (SEQ ID No: 2) 99.2 99.8 L244A / F432I (SEQ ID No: 3) 99.1 99.9 L143V / L244A / F432I (SEQ ID No: 4) 99.5 99.9 L244G / F277V / F432L (S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com