One-pot method for preparing hydrophilic phytosterol/stanol derivatives
A technology for phytostanols and phytosterols is applied in the field of preparing hydrophilic phytosterol/stanol derivatives, which can solve the problems of long reaction time and low product conversion rate, and achieves low cost, high product purity and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
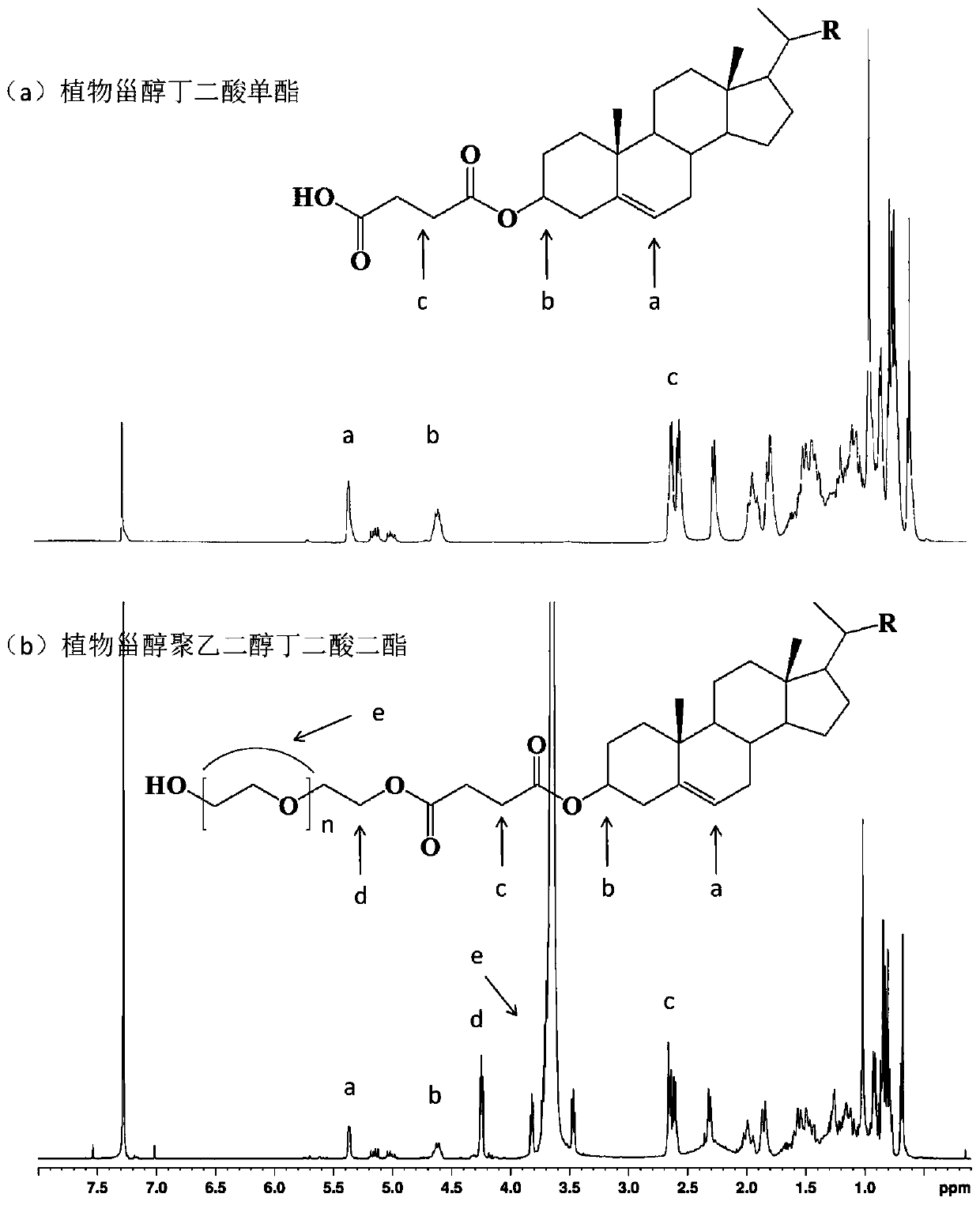
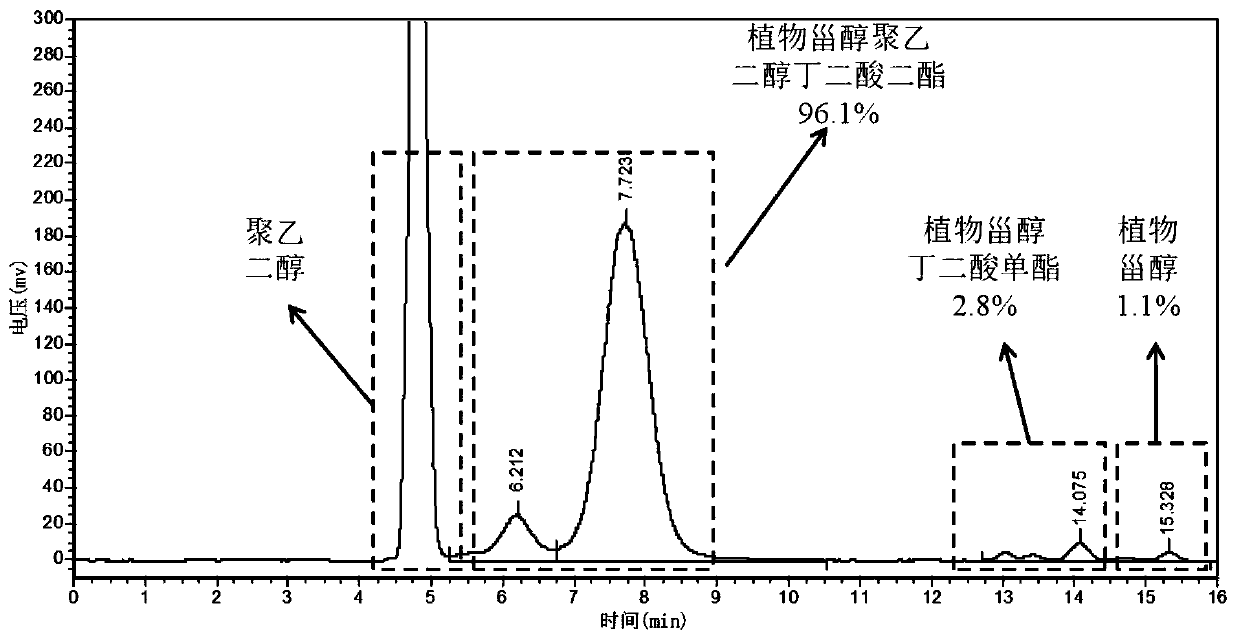
[0038] Example 1 Phytosterol polyethylene glycol succinate diester
[0039] 1. Preparation of phytosterol succinic acid monoester
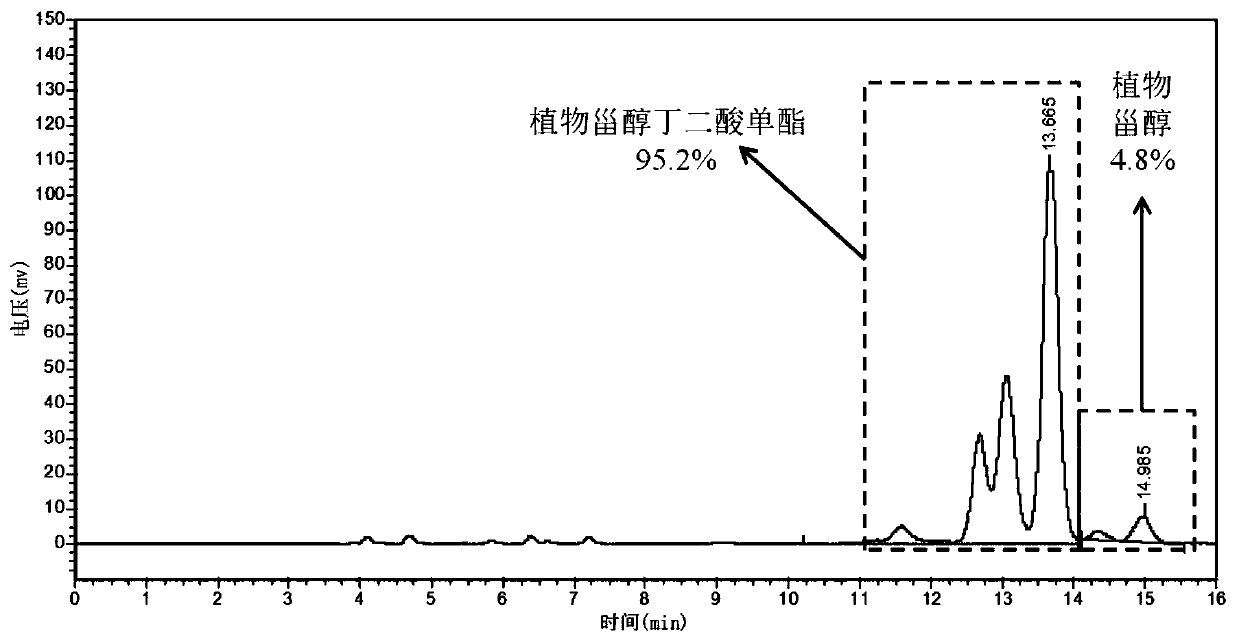
[0040] Preparation method: 12.4g of phytosterol, 3.0g of succinic anhydride, and 0.25g of ionic liquid 1-butylsulfonic acid-3-methylimidazole hydrogen sulfate were respectively added to a reflux reaction device equipped with stirring and oil bath temperature control. , add 100 mL of petroleum ether, turn on stirring to adjust the temperature to 100-110 °C, and react for 1-1.5 h. The conversion rate of phytosterol succinic acid monoester detected by HPLC can reach 98.7%. 10 mL of distilled water was added to the reaction solution, extracted, the organic phase layer was collected, and the solvent was removed by rotary evaporation to obtain 14.9 g of phytosterol succinic acid monoester.
[0041] Structural identification: Phytosterol succinate monoester FT-IR: 2370-3700cm -1 The broad peak between is the stretching vibration absorption of hydroxyl...
Embodiment 2
[0056] Example 2 Phytostanol tea polyphenol adipate diester
[0057] 12.5g of phytostanol, 21.9g of adipic acid, 1.0g of ionic liquid 1-propylsulfonic acid-3-methylimidazole p-toluenesulfonate were respectively added to the reflux reaction equipped with stirring and oil bath temperature control. device, add 200 mL of tert-butanol, turn on stirring to adjust the temperature to 80°C to 85°C, and react for 2.5h to 3h. Heating was stopped and 100 μL was sampled for HPLC analysis. The conversion rate of the intermediate product phytostanol adipate monoester can reach 95.3%.
[0058] After sampling, add 41.2g of hydrophilic modifier tea polyphenol, supplement 0g of ionic liquid 1-propylsulfonic acid-3-methylimidazole p-toluenesulfonate, adjust the temperature to 80℃~85℃, and continue the reaction for 5h~6h. Heating was stopped and 100 μL was sampled for HPLC analysis. The conversion rate of the final product phytostanol tea polyphenol adipate diester can reach 92.9%.
[0059] Af...
Embodiment 3
[0060] Example 3 Phytosterol maltitol suberic acid diester
[0061] 12.4g of phytosterol, 7.8g of suberic acid, and 0.36g of ionic liquid 1-butylsulfonic acid-3-methylimidazole trifluoromethanesulfonate were respectively added to the reflux reaction device equipped with stirring and oil bath temperature control. , add 100 mL of tert-amyl alcohol, turn on stirring to adjust the temperature to 100 ℃ ~ 105 ℃, and react for 1 h to 1.5 h. Heating was stopped and 100 μL was sampled for HPLC analysis. The conversion rate of the intermediate product phytosterol suberic acid monoester can reach 97.1%.
[0062] After sampling, add 20.6g of hydrophilic modifier maltitol, and supplement 0.24g of ionic liquid 1-butylsulfonic acid-3-methylimidazole trifluoromethanesulfonate, adjust the temperature to 100℃~105℃, and continue the reaction for 2h~ 3h. Heating was stopped and 100 μL was sampled for HPLC analysis. The conversion rate of the final product phytosterol maltitol suberic acid die...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com