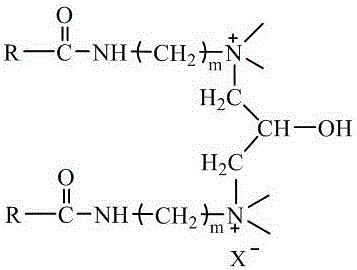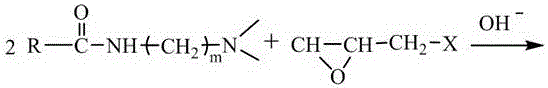Long-chain cationic gemini surfactant and preparation method thereof
A gemini surface and cationic technology, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, and the preparation of organic compounds, etc., can solve problems such as the impact of gemini surfactant application, complicated separation and purification process, and corrosion of production equipment, and reduce the Synthetic cost, increase reaction conversion rate, and reduce the effect of equipment corrosion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
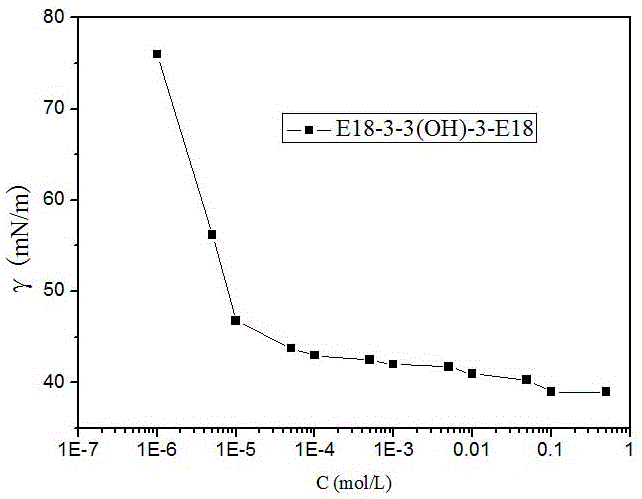
[0054] The long-chain cationic gemini surfactant E18-3-3(OH)-3-E18 has the following molecular structure:
[0055]
[0056] Its preparation method is:
[0057] Oleamidopropyldimethylamine (40mmol, 14.96g), epichlorohydrin (20mmol, 1.85g), potassium hydroxide (or sodium hydroxide) 0.15-0.20g were placed in a reaction flask, and reacted at 80°C for 6h, Added ethanol to wash, and after drying, 14.65 g of the product was obtained with a yield of 90.9%. After MS, 1 The H NMR identification product was consistent with the molecular structure of the target compound.
[0058] When Cl in the above molecular structure is replaced by Br, the epichlorohydrin in the preparation method is changed to epibromohydrin.
Embodiment 2
[0060] The long-chain cationic gemini surfactant E18-2-3(OH)-2-E18 has the following molecular structure:
[0061]
[0062] Its preparation method is:
[0063]Oleamide ethyl dimethylamine (40mmol, 14.48g), epichlorohydrin (20mmol, 1.85g), potassium hydroxide (or sodium hydroxide) 0.15-0.20g were placed in a reaction flask, and reacted at 70°C for 5h, Added ethanol to wash, and after drying, 14.19 g of the product was obtained with a yield of 91.2%. After MS, 1 The H NMR identification product was consistent with the molecular structure of the target compound.
[0064] When Cl in the above molecular structure is replaced by Br, the epichlorohydrin in the preparation method is changed to epibromohydrin.
Embodiment 3
[0066] Long-chain cationic gemini surfactant E22-3-3(OH)-3-E22 has the following molecular structure:
[0067]
[0068] Its preparation method is:
[0069] Erucic acid amidopropyl dimethylamine (40mmol, 16.88g), epichlorohydrin (20mmol, 1.85g), potassium hydroxide (or sodium hydroxide) 0.15-0.20g were placed in a reaction flask, and reacted at 90°C for 8h, Added ethanol to wash, and after drying, 16.31 g of the product was obtained with a yield of 90.4%. After MS, 1 The H NMR identification product was consistent with the molecular structure of the target compound.
[0070] When Cl in the above molecular structure is replaced by Br, the epichlorohydrin in the preparation method is changed to epibromohydrin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com