A sucrose fatty acid ester embedded type cationic liposome genetic vector system, a preparing method thereof and applications of the method
A technology of sucrose fatty acid ester and cationic liposome, which is applied in the field of sucrose fatty acid ester embedded cationic liposome gene carrier system and its preparation, can solve the problem of restricting the development of cationic liposome, unclear gene transfer mechanism, organ Targeting is not obvious and other problems, to achieve the effect of a wide range of hydrophilic and lipophilic balance, good biodegradability, and enhanced compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
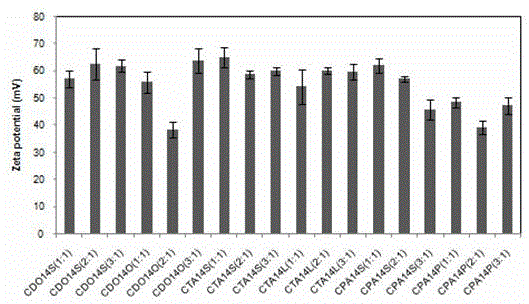
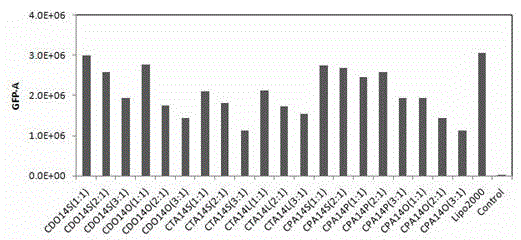
[0055] The preparation of embodiment 1 cationic liposome CDO14S
[0056] Weigh 1mg of peptide-type cationic lipid CDO14 and colipoid sucrose stearate S070 (1mg) in the same mass ratio, dissolve in 1mL methanol and chloroform mixed solvent (mixing ratio is 2:1 volume ratio), and wait for After fully dissolving, blow it into a uniform film under nitrogen, and dry it in vacuum for 5 hours to evaporate all the solvent (vacuum degree is -0.09MPa, normal temperature). After dissolving with 100 μL absolute ethanol, add 900 μL ultrapure water to make the liposome 1mg / mL, hydrated at about 80°C for 1h, ultrasonically oscillated at an ultrasonic frequency of 100Hz until clear and transparent, and prepared sucrose ester-embedded cationic liposomes with a concentration of 1mL / mg.
Embodiment 2
[0057] The preparation of embodiment 2 cationic liposome CTA14S
[0058] Weigh 0.5 mg of Gemini cationic lipid CTA14 and dissolve it in 1 mL of chloroform, add 1 mg of colipoid sucrose stearate S570 (mass ratio of CTA14 to S570 is 1:2), after fully dissolved, blow it under nitrogen Form a uniform film, vacuum dry for 12 hours to volatilize all the solvent (vacuum degree is -0.09MPa, room temperature), soak in 1mL ultrapure water for 2 hours to make the film fall off, and repeat ultrasonic vibration at about 55°C (oscillation frequency: 100Hz) until clear and transparent , to prepare sucrose ester embedded cationic liposomes with a concentration of 0.5 mg / mL.
Embodiment 3
[0059] The preparation of embodiment 3 cationic liposome CPA14P
[0060] Weigh 5 mg of quaternary ammonium cationic lipid CPA14 and 0.625 mg of colipoid sucrose palmitate P170 (the mass ratio of CPA14 to P-170 is 8:1) and dissolve it in 5 mL of chloroform. Blow down to form a uniform film, vacuum dry for 8 hours to evaporate all the solvent (vacuum degree is -0.09MPa, room temperature), add 5mL phosphate buffer, hydrate at 30°C for 8 hours, and ultrasonically oscillate at an ultrasonic frequency of 100Hz until clear and transparent. A concentration of 5 mg / mL sucrose ester-embedded cationic peptide liposomes was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com