Iron and vanadium synergistically doped lithium-rich manganese-based positive electrode material and preparation method thereof
A cathode material, lithium-rich manganese-based technology, applied in the field of lithium-rich manganese-based cathode materials and its preparation, can solve the problems of poor cycle performance, low initial storage efficiency, etc., achieve convenient operation, improve discharge specific capacity and initial coulombic efficiency , the effect of good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
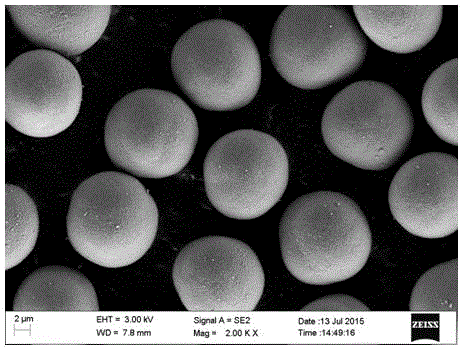
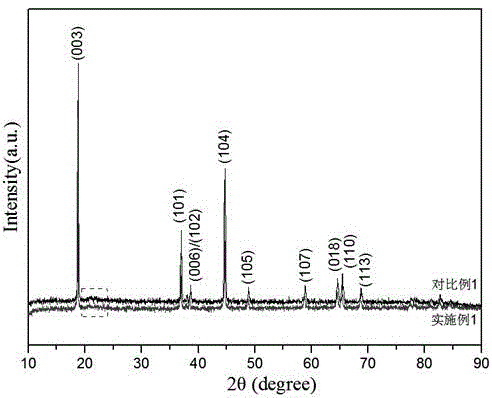
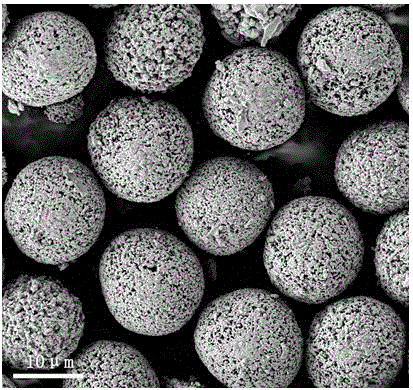
[0029] (1) Add 700mL of 0.1mol / L (NH 4 ) 2 SO 4 solution as the reaction bottom solution; the soluble sulfate MnSO 4 ·H 2 O. CoSO 4 ·7H 2 O, NiSO 4 ·6H 2 O is dissolved in deionized water at a molar ratio of 0.46:0.2:0.2 to prepare a 2mol / L mixed salt solution; 0.2mol / L ammonia water is used as a complexing agent, and 2mol / L sodium carbonate solution is used as a precipitating agent , pump all three into a tank containing (NH 4 )2SO 4 In the reaction kettle for the reaction bottom liquid, the stirring speed is controlled to 600rpm, the temperature of the reaction kettle is controlled at 50°C, the flow rate of the precipitant is controlled by the online pH automatic control system, so that the pH value of the reaction system is at 7.7, and the mixed salt solution is controlled at the same time The flow rate of the complexing agent is 1.5mL / min, the flow rate of the complexing agent is 1mL / min, and the reaction time is 5h. The carbonate coprecipitation precursor is prep...
Embodiment 2
[0034] (1) Add 500mL of 0.05mol / L (NH 4 ) 2 SO 4 The solution is used as the reaction bottom solution; the soluble nitrate Mn(NO 3 ) 2 , Co(NO 3 ) 2 ·6H 2 O, Ni(NO 3 ) 2 ·6H 2 O is dissolved in deionized water at a molar ratio of 0.54:0.13:0.13 to prepare a 3mol / L mixed salt solution; 0.5mol / L ammonia water is used as a complexing agent, and 3mol / L sodium bicarbonate solution is used as a precipitate Agent, pump the three into the containing (NH 4 ) 2 SO 4 In the reaction kettle for the reaction bottom liquid, the stirring speed is controlled at 2000rpm, the temperature of the reaction kettle is controlled at 40°C, the flow rate of the precipitant is controlled by the online pH automatic control system, so that the pH value of the reaction system is at 8.5, and the mixed salt solution is controlled at the same time The flow rate of the complexing agent is 2mL / min, the flow rate of the complexing agent is 1.5mL / min, and the reaction time is 4h. The carbonate co-prec...
Embodiment 3
[0038] (1) Add 1000mL0.01mol / L NH to the reactor 4 HCO 3 solution as the reaction bottom solution; the soluble acetate C 4 h 6 MnO 4 4H 2 O, C 4 h 6 NiO 4 4H 2 O, C 4 h 6 CoO 4 4H 2 O is dissolved in deionized water at a molar ratio of 0.50:0.167:0.167 to prepare a 1mol / L mixed salt solution; 0.2mol / L ammonia water is used as a complexing agent, and 1mol / L sodium carbonate solution is used as a precipitating agent , pump all three into the NH-containing 4 HCO 3 In the reaction kettle for the reaction bottom liquid, the stirring speed is controlled to 400rpm, the temperature of the reaction kettle is controlled at 80°C, and the flow rate of the precipitant is controlled by the online ph automatic control system, so that the pH value of the reaction system is 11.5, and the mixed salt solution is controlled at the same time The flow rate of the complexing agent is 2mL / min, the flow rate of the complexing agent is 0.1mL / min, and the reaction time is 8h. The carbonate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com