Fc-binding protein, method for producing said protein, and antibody adsorbent using said protein, and methods for purifying and identifying antibody using said adsorbent
一种结合性、蛋白质的技术,应用在识别糖链有无附加于抗体,抗体吸附剂领域,能够解决难以控制抗体糖链、分析需要很多时间和劳力等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1Fc
[0127] Example 1 Preparation of Fc-binding protein or human FcγRIIIa expression vector
[0128] (1) Based on the amino acid sequence from glycine (Gly) at position 17 to glutamine (Gln) at position 192 in the amino acid sequence of human FcγRIIIa described in SEQ ID NO: 1, DNAworks method (Nucleic Acids Res., 30, e43, 2002), designed a nucleotide sequence that converts codons from the human type to the E. coli type. The designed nucleotide sequence is shown in SEQ ID NO: 2.
[0129] (2) In order to prepare a polynucleotide comprising the sequence described in SEQ ID NO: 2, an oligonucleotide comprising the sequence described in SEQ ID NO: 3 to 20 was synthesized, and using the aforementioned oligonucleotide, the following procedures were performed: Two-stage PCR.
[0130] (2-1) PCR in the first stage: Prepare a reaction solution having the composition shown in Table 1, heat-treat the reaction solution at 98° C. for 5 minutes, then perform the first step at 98° C. for 10 seco...
Embodiment 2
[0140] Example 2 Introduction of mutations into Fc-binding protein and preparation of gene library
[0141] For the polynucleotide portion encoding the Fc-binding protein in the Fc-binding protein expression vector pER-eFcR prepared in Example 1, mutations were randomly introduced by error-prone PCR.
[0142] (1) Error-prone PCR was performed using pET-eFcR prepared in Example 1 as a template. Error-prone PCR was performed as follows: After preparing a reaction solution having the composition shown in Table 3, the reaction solution was heat-treated at 95°C for 2 minutes, the first step was performed at 95°C for 30 seconds, and the first step was performed at 60°C for 30 seconds. The second step of 1 second, the third step of 90 seconds at 72°C, these 3 steps were carried out as one cycle of reaction for 35 cycles, and finally heat treatment was performed at 72°C for 7 minutes. Mutations were favorably introduced into the polynucleotide encoding the Fc-binding protein by the a...
Embodiment 3
[0147] Example 3 Screening of thermostabilized Fc-binding proteins
[0148] (1) Inoculate the random mutant gene library (transformant) prepared in Example 2 into 2YT liquid medium (16 g / L of peptone, 10 g / L of yeast extract, chlorinated Sodium 5g / L) 200 μL, using a 96-well deep-well plate, shaking culture at 30°C for one night.
[0149] (2) After culturing, 5 μL of the culture solution was continuously implanted into 500 μL of IPTG (isopropyl-β-D-thiogalactopyranoside, isopropyl-β-D-thiogalactopyranoside) containing 0.05 mM, 0.3% glycine and In a 2YT liquid medium containing 50 μg / mL kanamycin, a 96-well deep-well plate was used, and cultured at 20° C. overnight with shaking.
[0150] (3) After culturing, the culture supernatant obtained by centrifugation was diluted 2-fold with 20 mM Tris hydrochloride buffer (pH 7.4) containing 150 mM sodium chloride. For the diluted solution, heat treatment was performed at 45°C for 10 minutes.
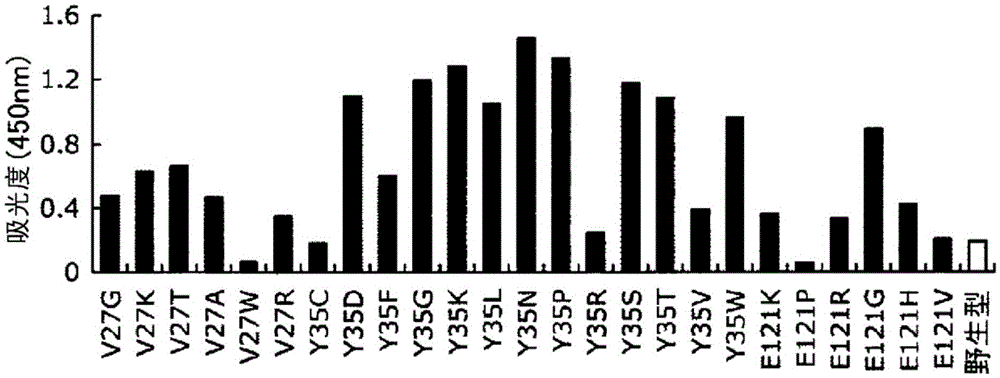
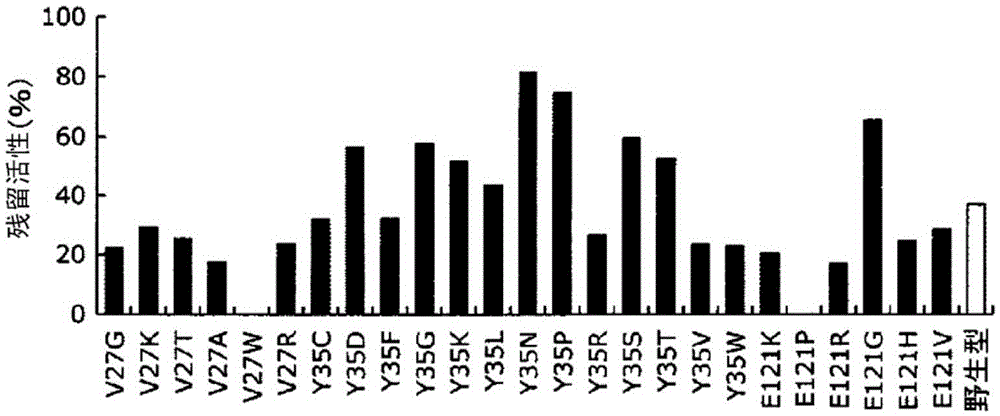
[0151] (4) The antibody-binding activity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com