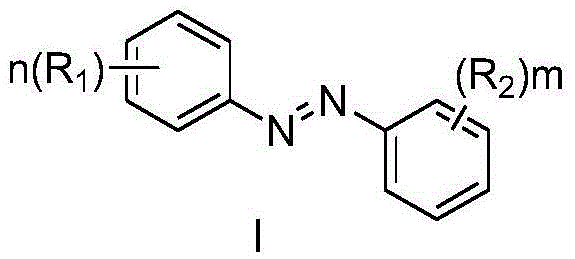
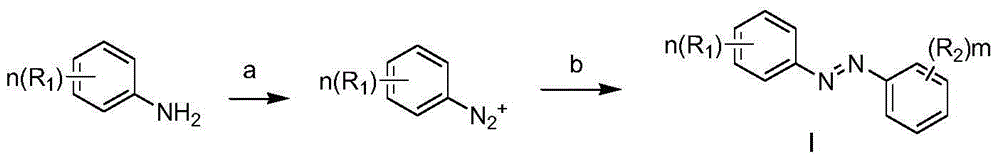
Azobenzene derivatives and their preparation method, pharmaceutical composition and use
A technology of azobenzene derivatives and azobenzene, which is applied in drug combinations, antipyretics, antitumor drugs, etc., to achieve high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1. (E)-4,4'-bis(2-methoxy n-propionic acid methyl ester-3-yl)azobenzene
[0059]
[0060] Methyl 3-p-aminophenyl-2-methoxy n-propionate (1.4g, 6.3mmol) was dissolved in 40mlTHF, the air was removed, sodium iodide (2.0g, 13mmol), t-BuOCl (1.4g, 13mmol), reacted under nitrogen protection for 3h, quenched with 1N sodium thiosulfate solution, extracted with ethyl acetate, washed the organic phase once with water, washed with saturated sodium chloride
[0061] Once, dried over anhydrous sodium sulfate, filtered and chromatographed to obtain 660 mg of an orange solid. 1HNMR (400MHz, DMSO-d 6 )δ:7.83~7.81(d,4H,ArH),7.43-7.41(d,4H,ArH),4.17~4.14(m,2H,-OCH-),3.66(s,6H,2-COOCH 3 -),3.29(s,6H,2-OCH 3 -),3.11~3.06(m,2H,-CH 2 -),3.04~2.85(m,2H,-CH 2 -).MS(FAB):m / z415[M+H] + .
Embodiment 2
[0062] Example 2. (E)-4,4'-bis(2-methoxyethyl n-propionate-3-yl)azobenzene
[0063]
[0064] With reference to Example 1, replace the compound 3-p-aminophenyl-2-methoxy n-propionate methyl ester with 3-p-aminophenyl-2-methoxy n-propionate ethyl ester (6.3mmol), the same operation process (E)-4,4'-bis(2-methoxyethyl-3-yl-n-propionate)azobenzene was obtained as an orange solid. 1 HNMR (400MHz, DMSO-d 6 )δ: 7.83~7.81(d,4H,-ArH),7.46~7.44(d,4H,-ArH),4.14~4.08(m,6H,-2OCH-,-2COOCH 2 -),3.22(s,6H,-2OCH 3 -),3.09~2.99(m,4H,-2CH 2 -),1.16~1.13(t,6H,2CH 3 -).MS(FAB):m / z443[M+H] + .
Embodiment 3
[0065] Example 3. (E)-4,4'-bis(2-methoxy n-propionic acid-3-yl)azobenzene
[0066]
[0067] Dissolve (E)-4,4'-bis(2-methoxyethyl n-propionate-3-yl)azobenzene (0.26g, 0.6mmol) in methanol and water (10ml, 10:1) , add sodium hydroxide (0.12g, 3.0mmol), react at room temperature for 5h, remove the solvent, add 2N HCl to adjust the pH = 2, a solid precipitates, and suction filter to obtain 200mg of (E)-4,4'-bis(2-methanol) Oxy-n-propanoate-3-yl)azobenzene, orange solid. 1 HNMR (400MHz, DMSO-d 6 )δ: 12.87(s, 2H, 2COOH), 7.81~7.79(d, 4H, ArH), 7.46-7.44(d, 4H, ArH), 4.03~4.00(m, 2H, 2OCH-), 3.26(s, 6H,2-OCH 3 ),3.11~3.06(m,2H,-CH 2 -),3.00~2.95(m,2H,-CH 2 -).MS(FAB):m / z387[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com