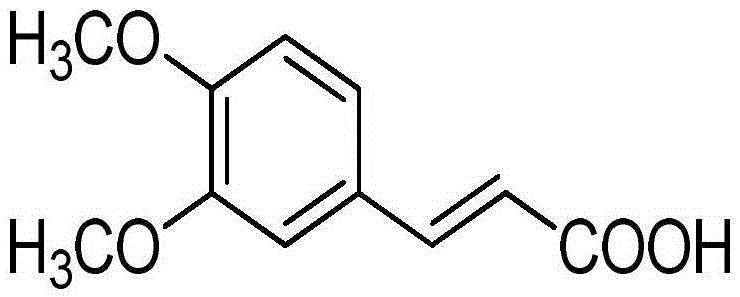
Application of methyl ferulic acid in preparation of medicine for preventing and treating alcoholic liver disease
A technology of methyl ferulic acid and alcoholic liver disease, applied in the field of medicine, can solve the problems of prevention and treatment that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
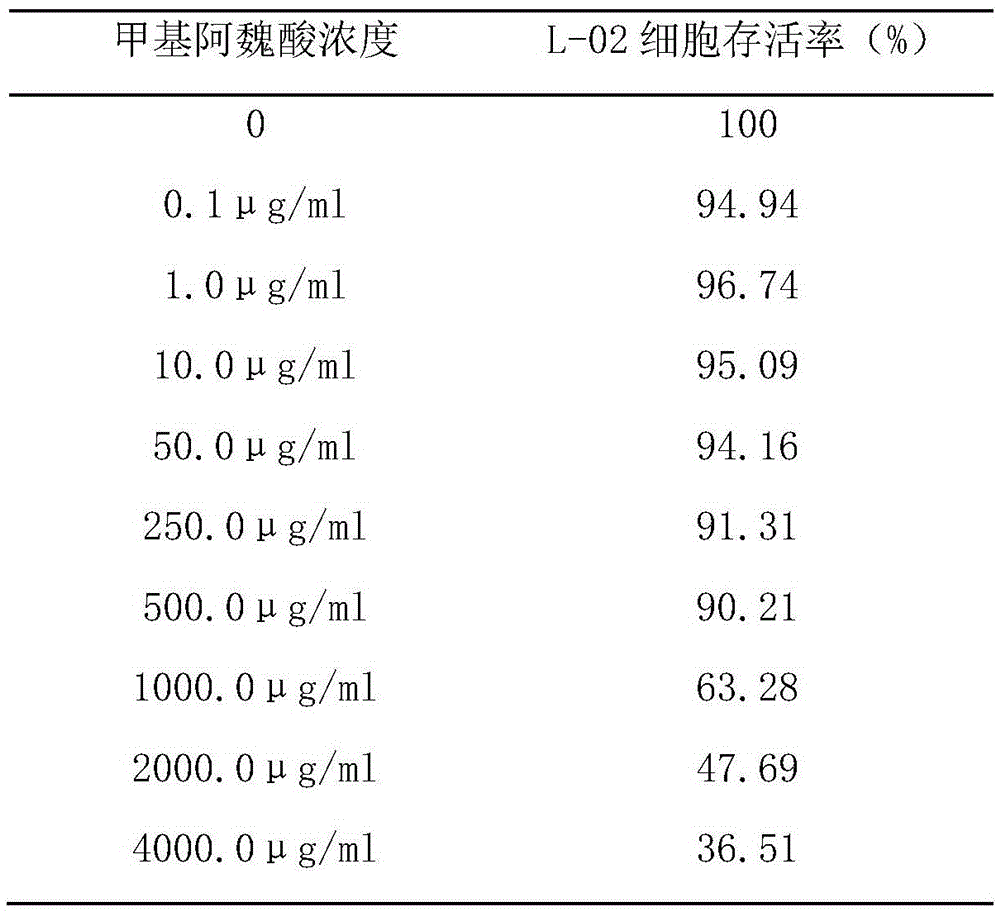
[0013] Example 1 Effect of methyl ferulic acid on ethanol-induced human normal liver cell line (L-02)
[0014] 1.1 Observation of different concentrations of methyl ferulic acid on the survival rate of L-02 cells.
[0015] Inoculate logarithmic phase cells in 96-well plate, 5000 cells per well, add a certain concentration of methyl ferulic acid after 24 hours, set 5 duplicate holes for each drug concentration, add MTT (5mg / ml) after 44 hours of drug addition Continue to incubate with 20ul for 4h, pour out the medium, blot dry, add 200ul of dimethyl sulfoxide to each well, mix well and then set an automatic microplate reader (detection wavelength 490nm) to read the optical density value (OD value) of each well and record the results , and calculate the survival rate. Survival rate (%)=(experimental group-blank control group) / (normal control group-blank control)×100%. The results are shown in Table 1.
[0016] Table 1. The effect of methyl ferulic acid on the survival rate of...
Embodiment 2
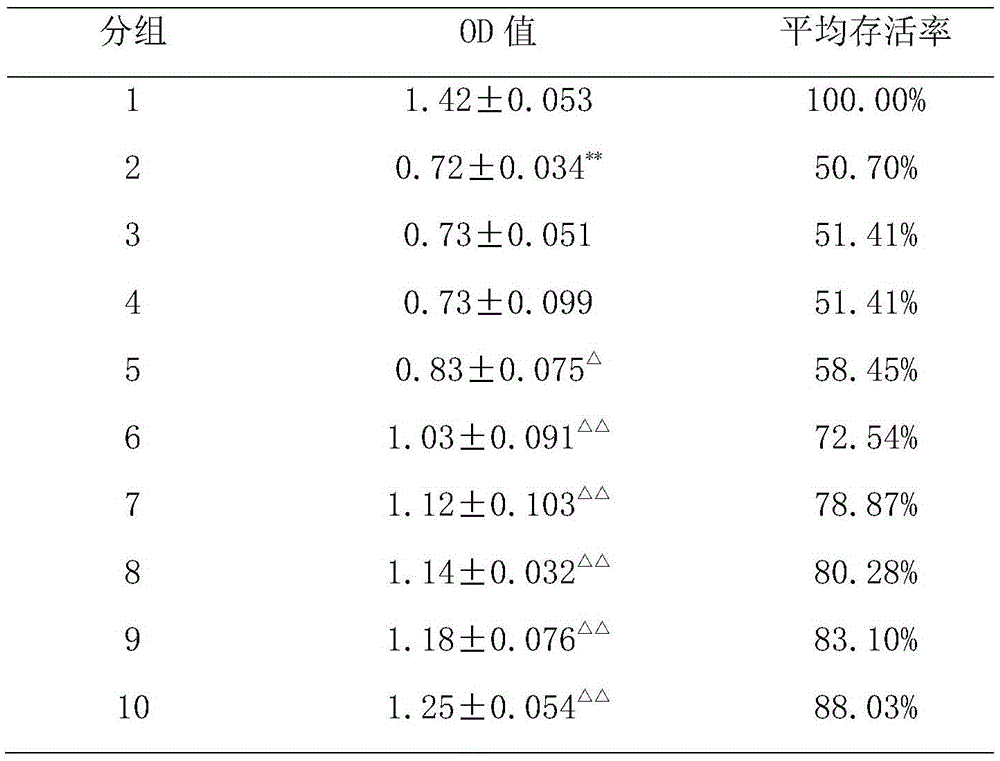
[0031] Example 2 The protective effect of methyl ferulic acid on the liver of acute alcoholic liver disease rat model:
[0032] 1. Drugs and animals
[0033] 1.1 Animals 60 SD rats, half male and half male, weighing 200±20g
[0034] 1.2 Grouping of animals: Randomly divide the animals into 6 groups, 10 in each group.
[0035] 1.3 Test drugs
[0036] (1) Bifendate, which is a traditional liver-protecting drug, is a drug approved by SFDA for marketing, and is used as a positive control drug;
[0037] (2) Methyl ferulic acid is a test drug to observe its protective effect on the liver of rat acute alcoholic liver disease model;
[0038] (3) Drug vehicle: 1% sodium carboxymethylcellulose solution.
Embodiment approach
[0040] The 1st group is the normal control group: intragastric administration of drug vehicle, the dose is 10ml / kg;
[0041] The 2nd group is the model control group: intragastric administration of drug solvent, the dose is 10ml / kg;
[0042]The third group is the bifendate treatment group: intragastric administration of bifendate at a dose of 200 mg / kg;
[0043] The 4th, 5th, and 6th groups were respectively low, medium, and high doses of methyl ferulic acid treatment groups: intragastric administration of methyl ferulic acid, and the doses were 10 mg / kg, 50 mg / kg, and 100 mg / kg respectively.
[0044] From the 4th day onwards, except the blank group, the rest of the groups were administered normally in the morning, 10ml / kg of 60% alcohol was administered in the afternoon, and the food and water were not limited. The alcohol was administered continuously for 10 days, and 1 hour after the end of the last oral administration of 60% alcohol. , anesthetized, and then blood and liv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com