Application of linear polythiourea and hyperbranched polythiourea in preparation of anti-tumor medicines and anti-virus or anti-bacterial medicines
An antibacterial drug, polythiourea technology, applied in the direction of antitumor drugs, antibacterial drugs, antiviral agents, etc., can solve the problems of complicated preparation process and strong toxicity of raw materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 utilizes 1,4-dithioisocyanate butane to react with ethylenediamine to prepare a linear thiourea polymer. The reaction formula is as follows:
[0039]
[0040] 1,4-Dithioisocyanate butane (0.69 g, 4 mmol) and ethylenediamine (0.24 g, 4 mmol) were mixed in 2 mL of dichloromethane, and stirred at room temperature for 24 hours. After three times of precipitation with ether, a colorless viscous product was obtained with a yield of 90% and a number average molecular weight of 14500.
[0041] The above obtained dope (0.7g) and PEG550-NH 2 (1 g) was dissolved in 5 mL of dichloromethane. After the reaction solution was stirred overnight at room temperature, it was precipitated three times with ether to obtain a PEGylated linear polymer containing thiourea (1.0 g).
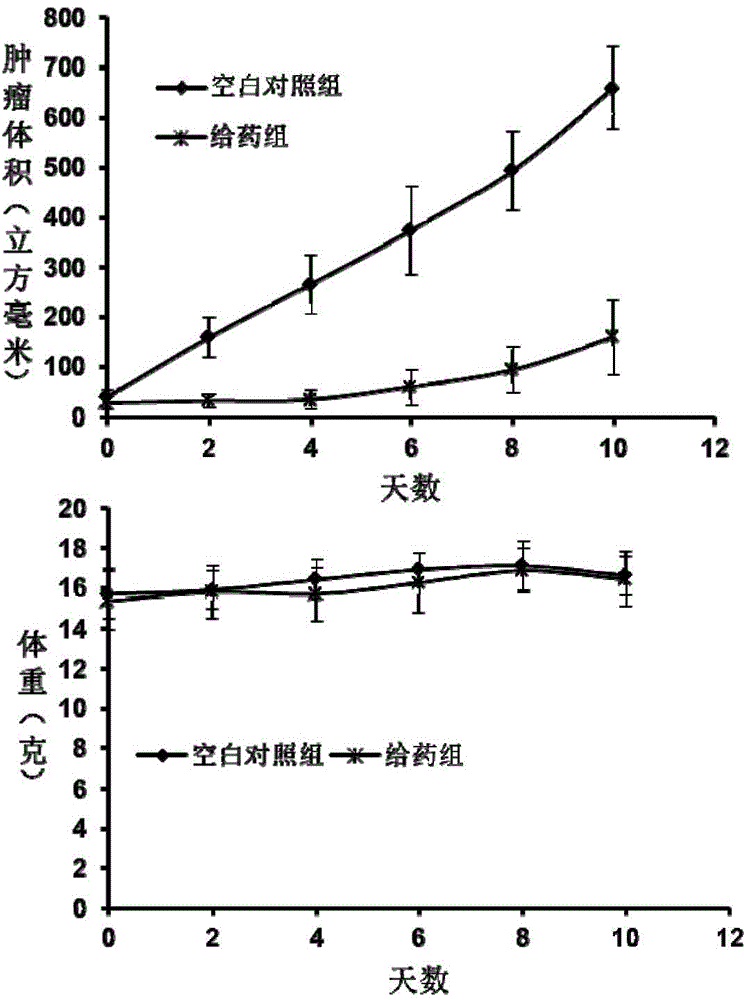
[0042] The pegylated thiourea-containing linear polymer synthesized in Example 1 was used to carry out an in vivo tumor inhibition test on the human colorectal cancer tumor model of nude mice, and a co...
Embodiment 2
[0045] Embodiment 2 utilizes 1,4-dithioisocyanate butane and three-(2-aminoethyl)amine to react to prepare thiourea-containing hyperbranched polymer
[0046] The reaction formula is as follows:
[0047]
[0048] 1,4-Dithioisocyanate butane (0.69 g, 4 mmol) and tris-(2-aminoethyl)amine (0.58 g, 4 mmol) were mixed in 3 mL of dichloromethane, and stirred at room temperature for 24 hours. After three times of precipitation with ether, a colorless viscous product was obtained with a yield of 93% and a number average molecular weight of 4500.
[0049] After PEG550-COOH (2g) was dissolved in 20mL of dichloromethane, carbonyldiimidazole (1g) was added, and after stirring at room temperature for 15min, the above-mentioned viscous material (0.5g) was added. After the reaction solution was stirred overnight at room temperature, it was precipitated with ether three times to obtain a PEGylated thiourea-containing hyperbranched polymer (1.2 g).
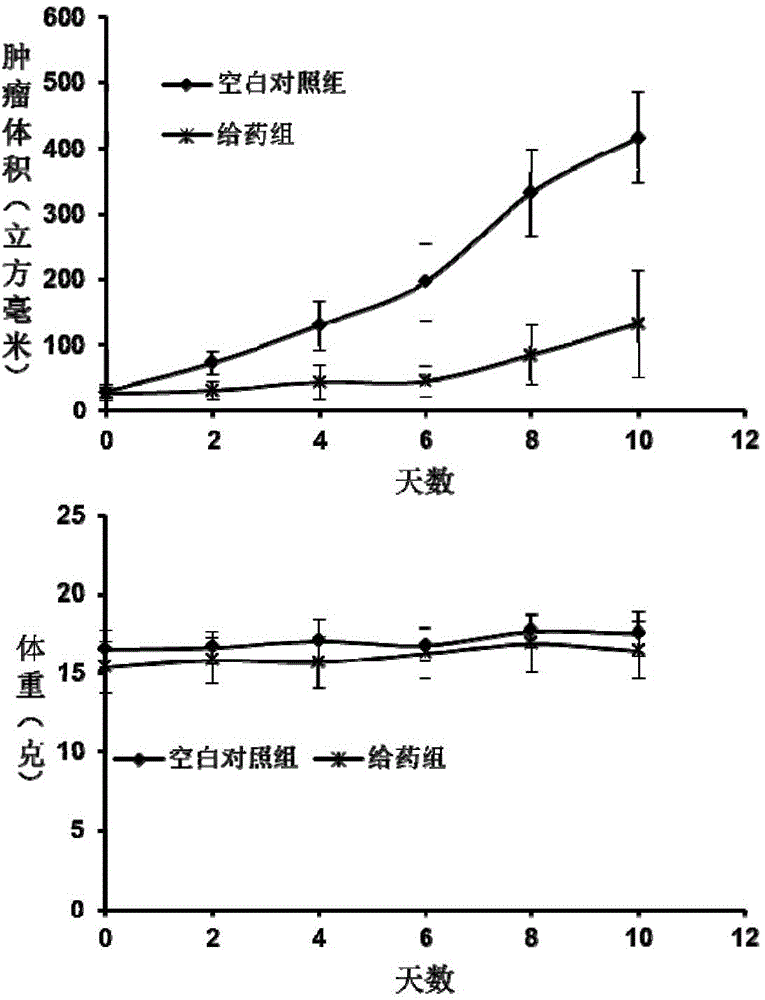
[0050] The thiourea-containing hyperbra...
Embodiment 3
[0051] Example 3 The linear thiourea polymer is prepared by reacting thiourea molecules with 2-hydroxyl-1,3-propanediamine. The reaction formula is as follows:
[0052]
[0053] 1.0000g of thiourea, 1.1839g of 1,3-diamino-2-hydroxypropane and 20mg of p-toluenesulfonic acid were dissolved in 5mL of NMP, and reacted overnight at 160°C under the protection of argon. After the product was precipitated with anhydrous ether, a small amount of After washing with ice water, 1.35 g of the final product represented by formula I was obtained with a yield of 76% and a number average molecular weight of 5000-6000.
[0054] 1 H-NMR (400MHz, DMSO): δ (ppm) 7.76 (s, 2H), 5.13 (d, J = 3.1Hz, 1H), 3.89 (d, J = 3.2Hz, 1H), 3.16 (d, J = 11.8Hz, 2H), 2.92 (d, J = 12.0Hz, 2H).
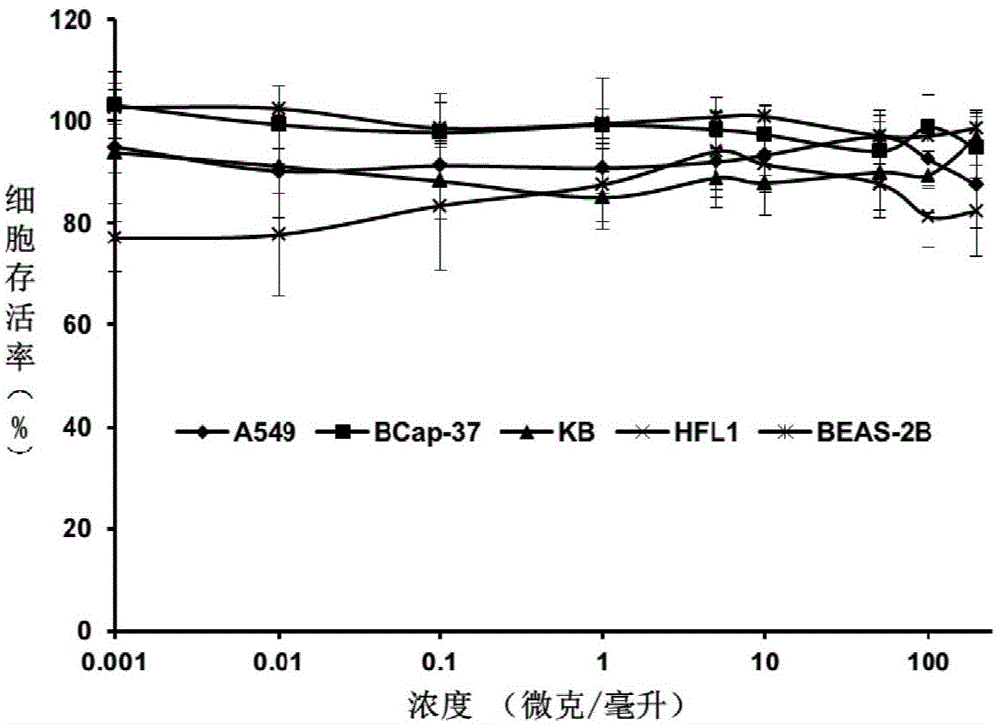
[0055] Taking the polythiourea molecule (abbreviated as L-PTU) prepared in Example 3 as an example, the MTT (3-(4,5-dimethylthiazole-2)-2,5 -diphenyltetrazolium bromide) method to detect the effect of L-PTU on human l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com