Ultraviolet spectrophotometry for polysaccharide content in medicine material fokien angiopteris rhizomes
A technology for polysaccharides and polysaccharide content of horseshoe fern, which is applied in the direction of color/spectral characteristic measurement, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] 2.1 Preparation of calla fern polysaccharide extract
[0024] A. Grind 1 kg of calla fern into coarse powder, add 8 L of 60% ethanol, heat and reflux twice for 2 hours each time, filter, discard the filtrate, collect medicinal residues, and remove fat-soluble components.
[0025] Add 0.3 mol / L dilute hydrochloric acid to 8 L of B medicinal residues, heat and reflux to extract twice, each time for 2 hours, filter, discard medicinal residues, collect the filtrate and concentrate to obtain 150 g of ointment.
[0026] Add ethanol to the C concentrated solution to make the ethanol concentration reach 80%, let stand overnight, filter, collect the precipitate, add water to dissolve the precipitate, filter, concentrate the filtrate into a paste, and vacuum freeze-dry to obtain the calla fern polysaccharide extract.
[0027] 2.2 Determination of polysaccharide content in calla fern polysaccharide extract
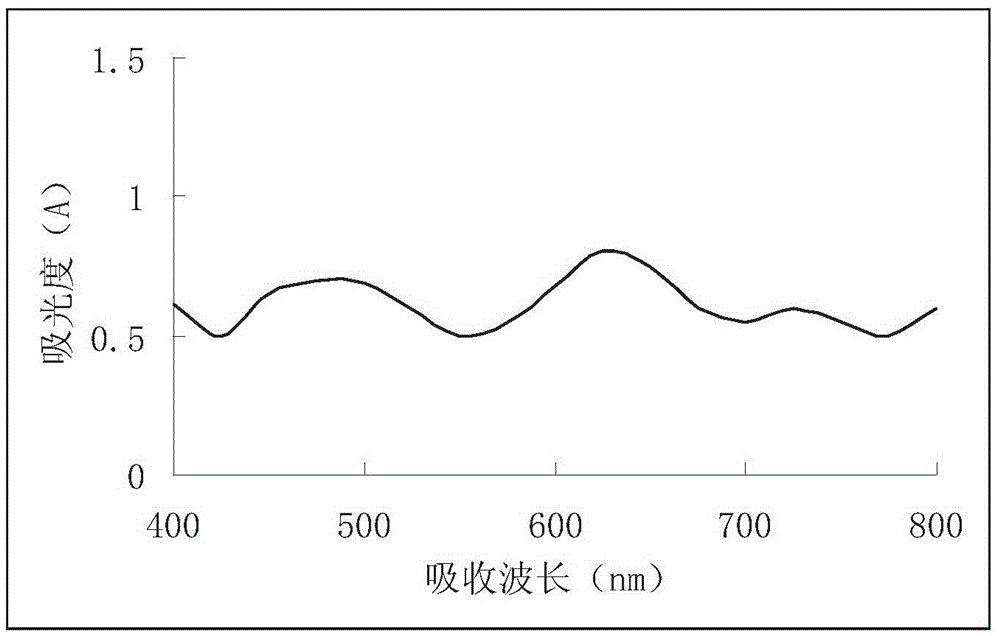
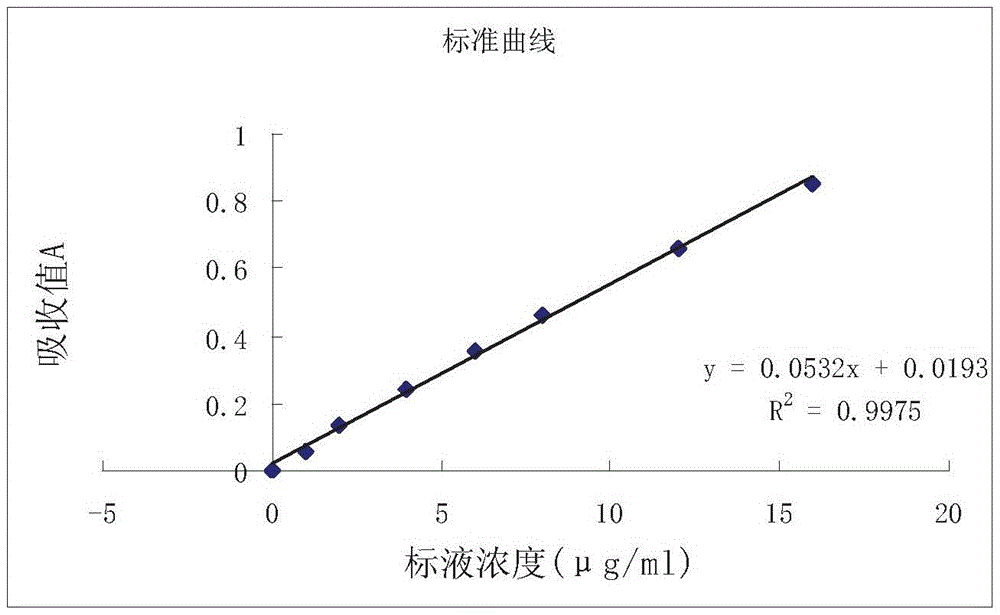
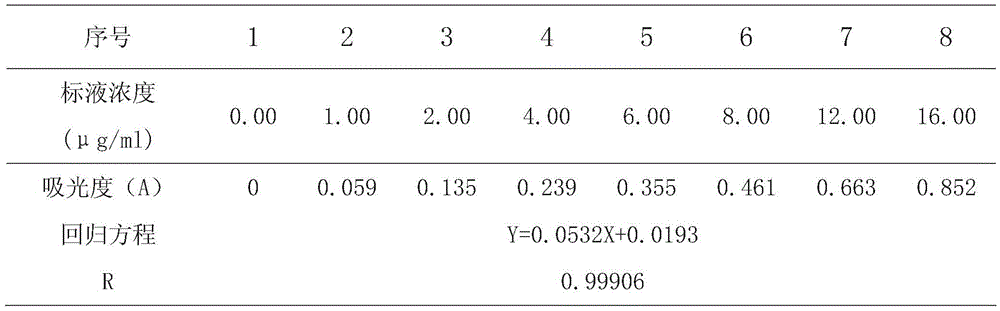
[0028] The calla fern polysaccharide extract is analyzed as the sample t...
Embodiment 1
[0042] Refer to the above assay method, but use 0.1% anthrone-sulfuric acid reagent to react and measure to obtain the polysaccharide content in the calla fern polysaccharide extract, and the results are shown in Table 3.
[0043] table 3
[0044] Sampling volume (ml)
Embodiment 2
[0046] Referring to the above assay method, but using 0.3% anthrone-sulfuric acid reagent for reaction and determination, the polysaccharide content in the calla fern polysaccharide extract was obtained, and the results are shown in Table 4.
[0047] Table 4
[0048] Sampling volume (ml)
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com